Find below an abstract of SeRMN contribution at 4th International DNP Symposium that will be held August 28-31, 2013, in Copenhagen (Denmak).
Enantiodiscrimination Studies by 13C DNP-NMR Spectroscopy
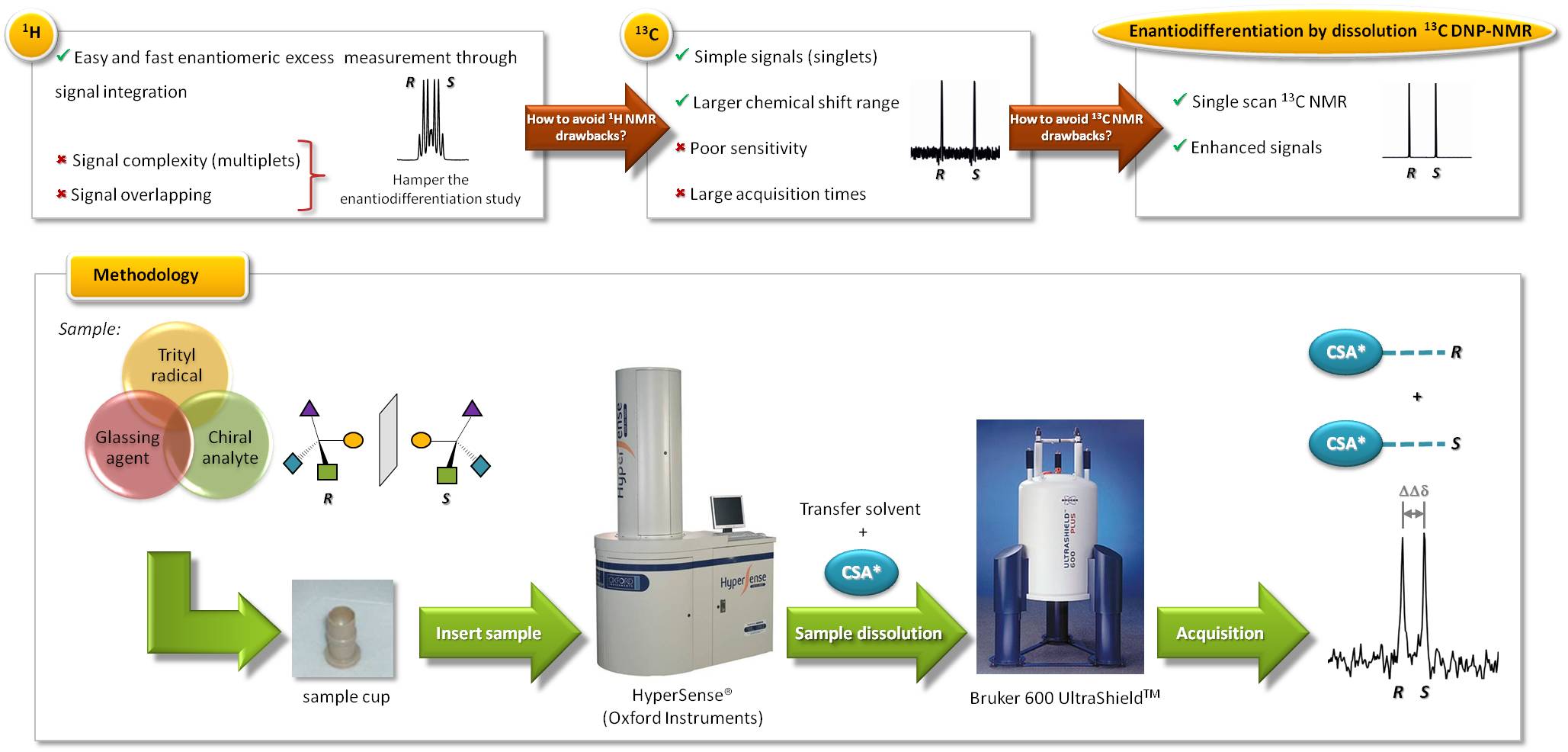
The determination of enantiomeric purity of drugs and/or endogenous molecules is crucial since its chirality could determine its pharmacological or biological behavior [1]. Many analytical techniques are available to determine the enantiomeric excess (ee) such as, circular dichroism, capillary electrophoresis, chromatographic techniques with chiral stationary phases, etc.; having each of them drawbacks and advantages [2]. Nuclear magnetic resonance (NMR) using a chiral solvating agent (CSA) as chiral auxiliary is an easy, fast and very powerful analytical tool that allows the measurement of ee by simple signal integration [3]. Typically, 1H NMR experiments are performed because of its high sensitivity and its presence in almost all organic molecules; however, both overcrowding and complexity of signals (multiplicity) often hampers the enantiomeric differentiation. The use of proton decoupled 13C NMR spectroscopy helps to avoid these drawbacks because of the simplicity of the signals obtained (singlets) and its larger chemical shift range (200 ppm). Unfortunately, the intrinsic low natural abundance of 13C nuclei (1.1%) means a poor sensitivity and usually too long acquisition times. Dissolution dynamic nuclear polarization (DNP) offers an elegant solution to sensitivity problems allowing obtaining single-scan 13C NMR spectra with signal enhancements of several orders of magnitude [4]. So far, no studies with chiral discrimination purposes have been performed taking advantage of the powerful technique of dissolution DNP.
The aim of this work is to combine the dissolution DNP technique and 13C NMR spectroscopy for the enantiodiscrimination of racemic analytes using a CSA due to its attractive properties. With this novel methodology a single-scan 13C NMR spectrum of a racemic analyte would be observed, where carbon NMR signals for each enantiomer are distinguished.
Here, we describe the hyperpolarization of an interesting a-amino acid such as methionine in a proper glassing matrix doped with trityl radical OX63. The hyperpolarized methionine is mixed with the transfer solvent containing the proper amount of (-)-(18-crown-6)-2,3,11,12-tetracarboxylic acid working as CSA. As a result, enhanced methionine signals would be observed in the corresponding 13C NMR spectrum, avoiding overlapping with both CSA and solvents signals.