Simões, Rui V., Miquel E. Cabañas, Carla Loreiro, Miriam Illa, Fatima Crispi & Eduard Gratacós. 2018. Assessment of prenatal cerebral and cardiac metabolic changes in a rabbit model of fetal growth restriction based on 13C-labelled substrate infusions and ex vivo multinuclear HRMAS. PLOS ONE 13(12). e0208784. DOI: 10.1371/journal.pone.0208784
Background: We have used a previously reported rabbit model of fetal growth restriction (FGR), reproducing perinatal neurodevelopmental and cardiovascular impairments, to investigate the main relative changes in cerebral and cardiac metabolism of term FGR fetuses during nutrient infusion.
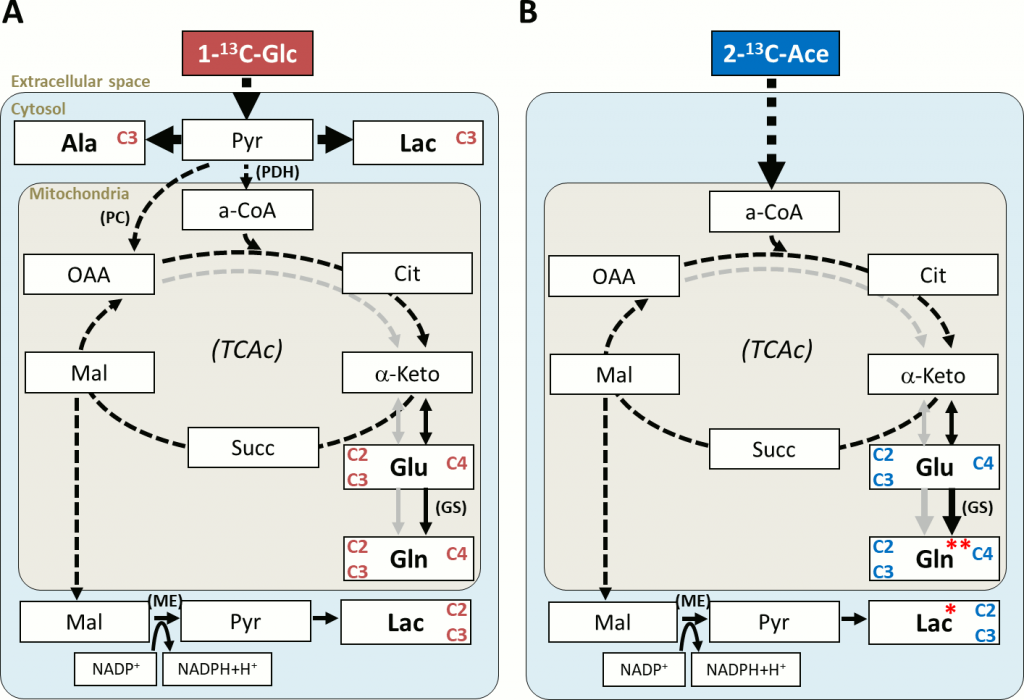
Methods: FGR was induced in 9 pregnant New Zealand rabbits at 25 days of gestation: one horn used as FGR, by partial ligation of uteroplacental vessels, and the contralateral as control (appropriate for gestation age, AGA). At 30 days of gestation, fasted mothers under anesthesia were infused i.v. with 1-13C-glucose (4 mothers), 2-13C-acetate (3 mothers), or not infused (2 mothers). Fetal brain and heart samples were quickly harvested and frozen down. Brain cortex and heart apex regions from 30 fetuses were studied ex vivo by HRMAS at 4°C, acquiring multinuclear 1D and 2D spectra. The data were processed, quantified by peak deconvolution or integration, and normalized to sample weight.
Results: Most of the total 13C-labeling reaching the fetal brains/hearts (80–90%) was incorporated to alanine and lactate (cytosol), and to the glutamine-glutamate pool (mitochondria). Acetate-derived lactate (Lac C2C3) had a slower turnover in FGR brains (~ -20%). In FGR hearts, mitochondrial turnover of acetate-derived glutamine (Gln C4) was slower (-23%) and there was a stronger accumulation of phospholipid breakdown products (glycerophosphoethanolamine and glycerophosphocholine, +50%), resembling the profile of non-infused control hearts.
Conclusions: Our results indicate specific functional changes in cerebral and cardiac metabolism of FGR fetuses under nutrient infusion, suggesting glial impairment and restricted mitochondrial metabolism concomitant with slower cell membrane turnover in cardiomyocytes, respectively. These prenatal metabolic changes underlie neurodevelopmental and cardiovascular problems observed in this FGR model and in clinical patients, paving the way for future studies aimed at evaluating metabolic function postnatally and in response to stress and/or treatment.