Last July I defended the doctoral thesis entitled “Nous agents de solvatació quiral tipus pinça amb anells antracènics: derivats de trifluorometilamines i trifluorometilcarbinols binòlics”.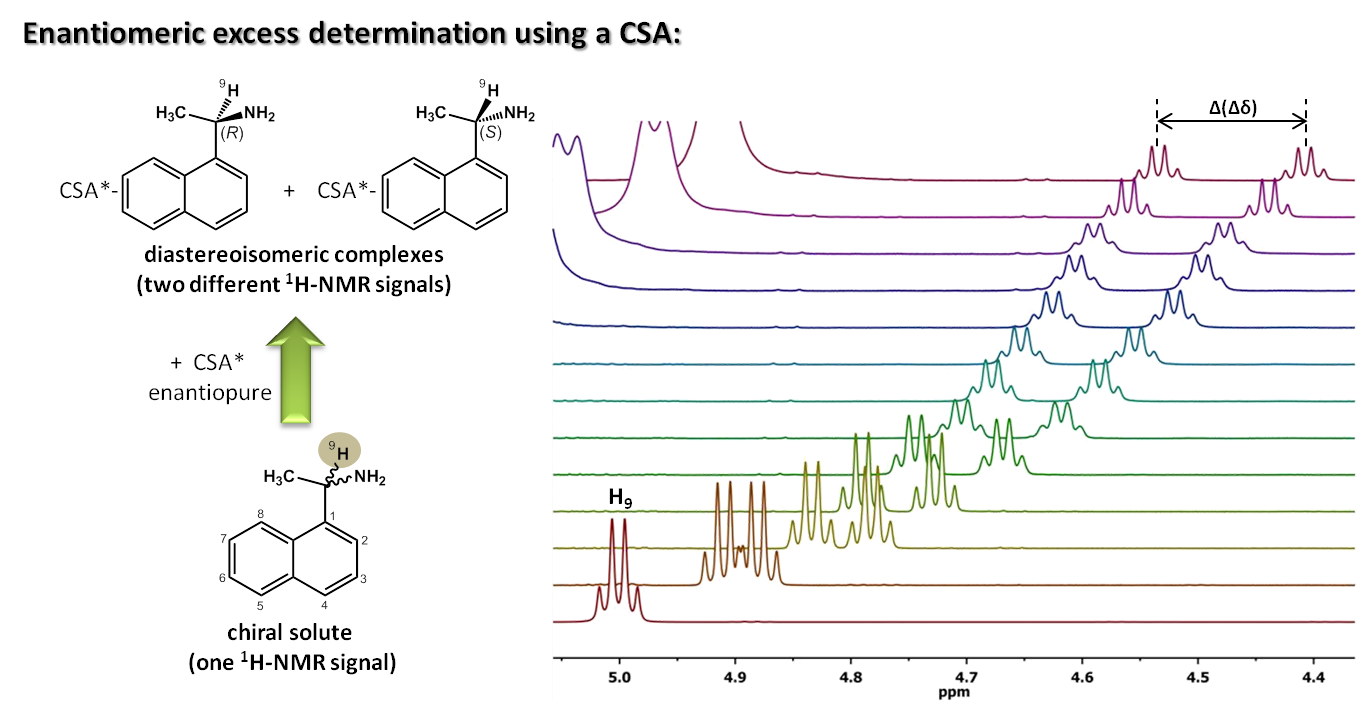
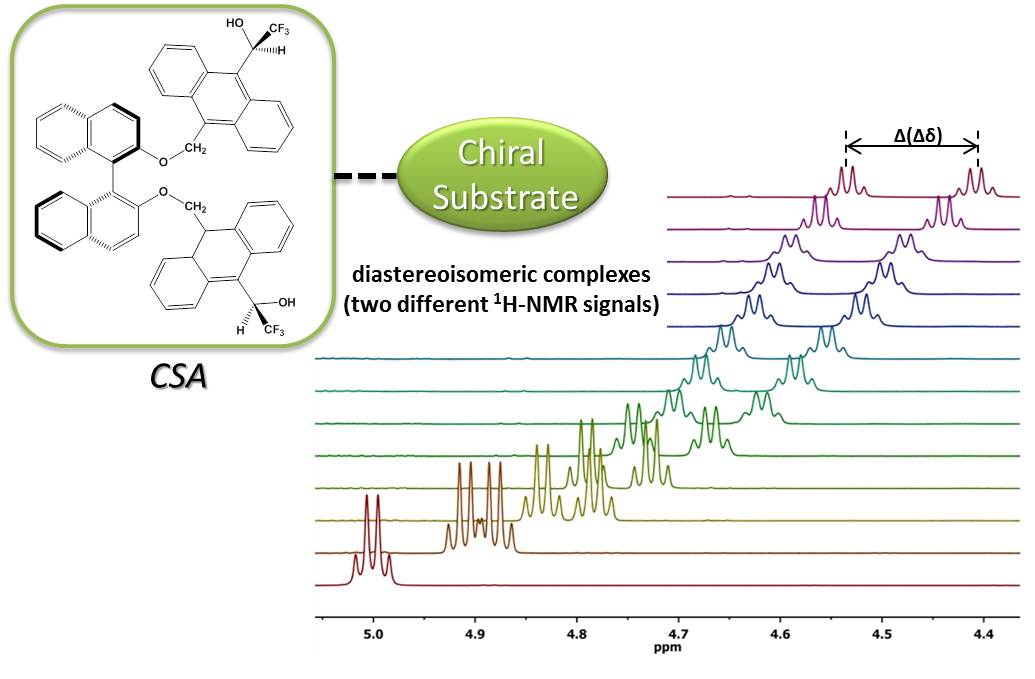
The thesis focuses on the synthesis and behaviour of new Chiral Solvating Agents (CSA) based on molecular tweezers. The newly synthesized enantiopure compounds can be classified in two big groups:
- the ones with a trifluoromethylanthrylamine backbone and an isopthalyc acid as a linking molecule, and
- the trifluoromethylcarbinol derivatives linked by a BINOL molecule, which adds an additional stereogenic element due to its chiral axis.
As CSA these compounds have to collect several main elements into its structure in order to be able to generate different diastereomeric complexes from each enantiomer in the substrate. Thus, it is essential for them to have the capability of generating non covalent interactions by means of complementarity in size, geometry and type of functional groups involved in the two interacting substances. It is very important, as well, that the CSA structure contains a very anisotropic group close to the chiral center. For instance, an anthracene ring, which will stimulate the formation of a non equivalent magnetic environment at the substrate stereogenic centers, favoring in this way the enantiorecognition.
The new compounds enantiodifferenciating capacity has been shown by measuring of enantiomeric excesses by means of NMR. Compounds have been evaluated by using different reference substrates, and some of the distereoisomeric complexes generated have been studied in depth.
The research work and the thesis defence were highly praised by the dissertation committee, and I was awarded a doctoral degree with honours (cum laude).