 “13C NMR spectroscopy for the differentiation of enantiomers using chiral solvating agents” Míriam Pérez-Trujillo, Eva Monteagudo and Teodor Parella. Analytical Chemistry, 2013, 85 (22), pp 10887–10894. DOI: 10.1021/ac402580j
“13C NMR spectroscopy for the differentiation of enantiomers using chiral solvating agents” Míriam Pérez-Trujillo, Eva Monteagudo and Teodor Parella. Analytical Chemistry, 2013, 85 (22), pp 10887–10894. DOI: 10.1021/ac402580j
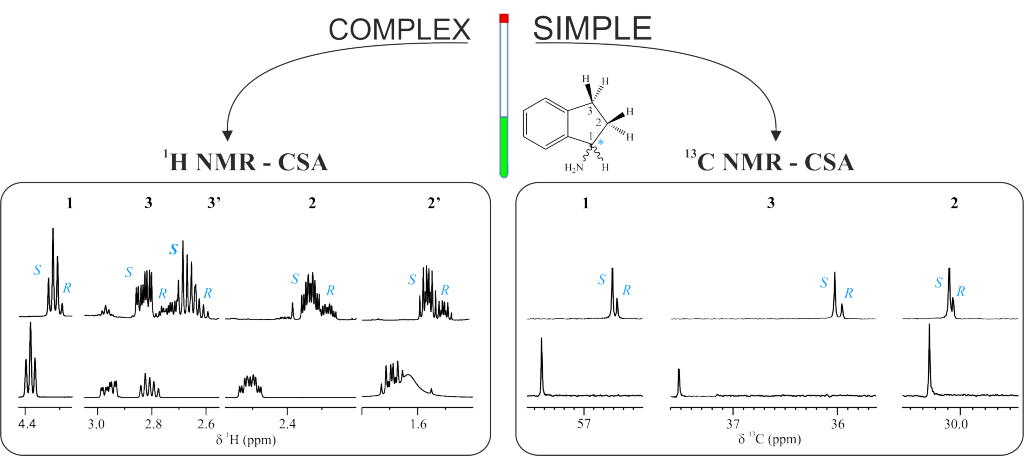
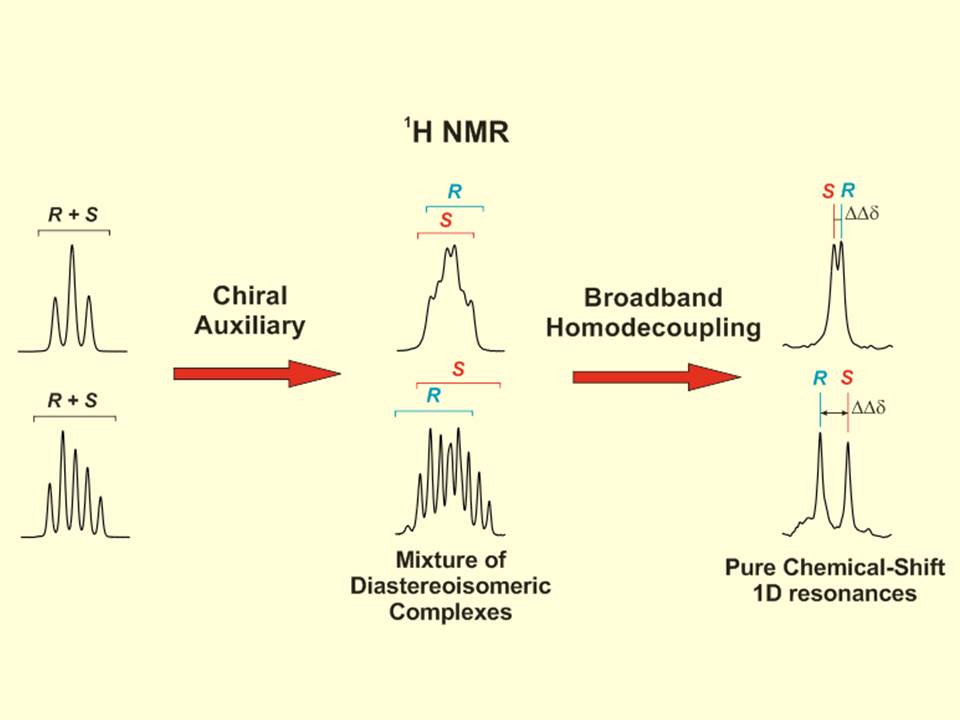
The utility of 13C NMR spectroscopy for the differentiation of enantiomers using chiral solvating agents (CSA) is stated. Three examples involving the enantiodifferentiation of a drug, a metabolite and a reactant in aqueous and organic solutions have been chosen to show it. The intrinsic high dispersion of 13C nucleus as well as the singlet nature of the signals in standard experiments makes 13C NMR experiments a powerful alternative and/or complement to 1H NMR experiments; specially, when studying pure compounds with complex proton spectra or mixtures of compounds, as in chiral metabonomics, where severe overlapping exists in proton spectrum. To evaluate and compare the quality of the enantioresolution of a NMR signal we introduce the enantiodifferentiation quotient, E, that considers the complexity of 1H multiplets (and in general the width) of the original signal. It has been observed that the error in the measurement of the enantiomeric molar ratio can be related to the E value. The sensitivity and experimental time of a wide range of chiral analyte concentrations was also assessed.