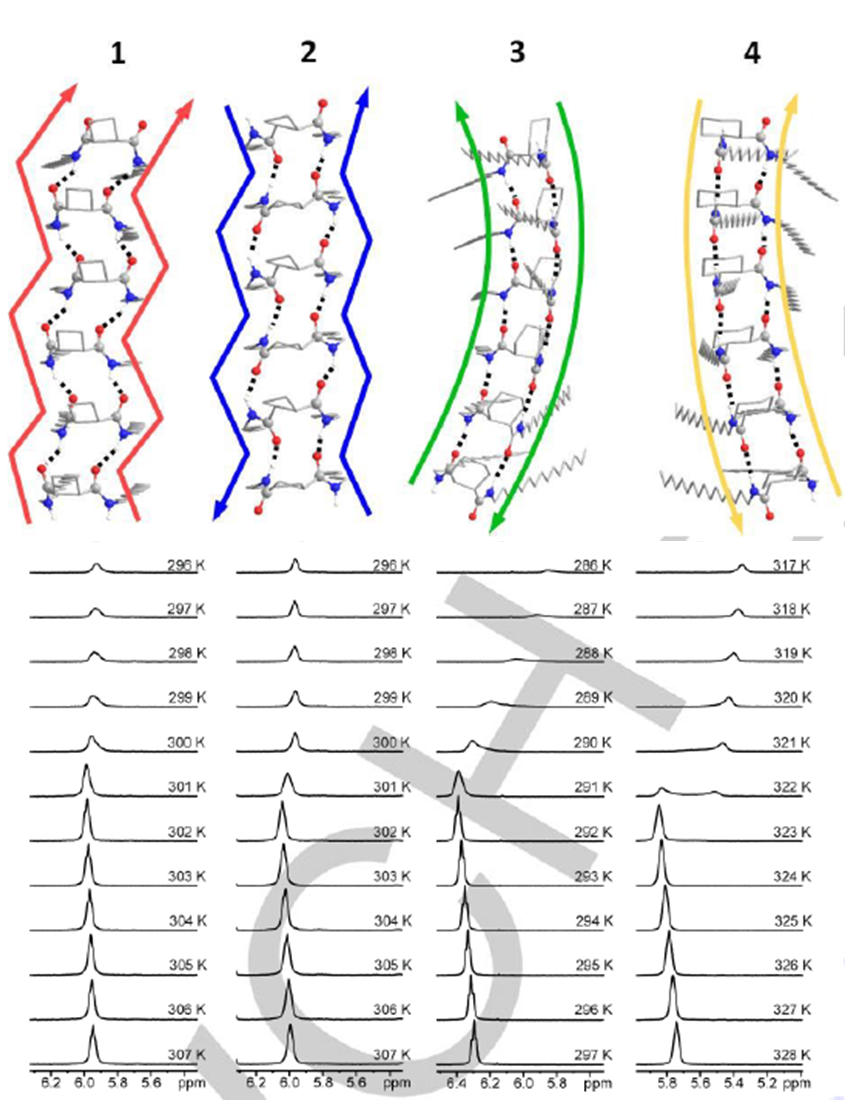
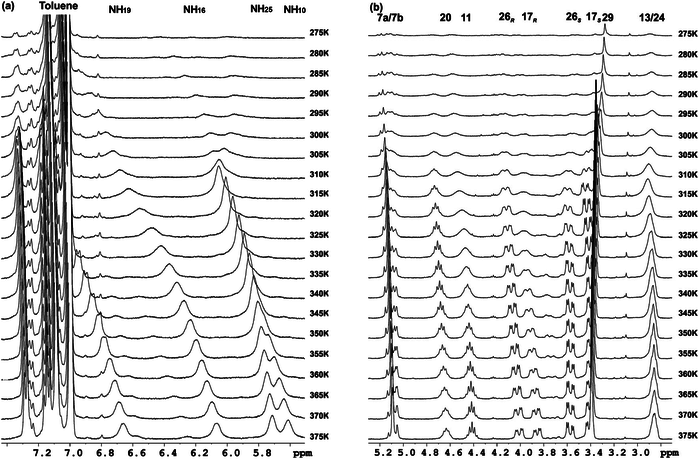
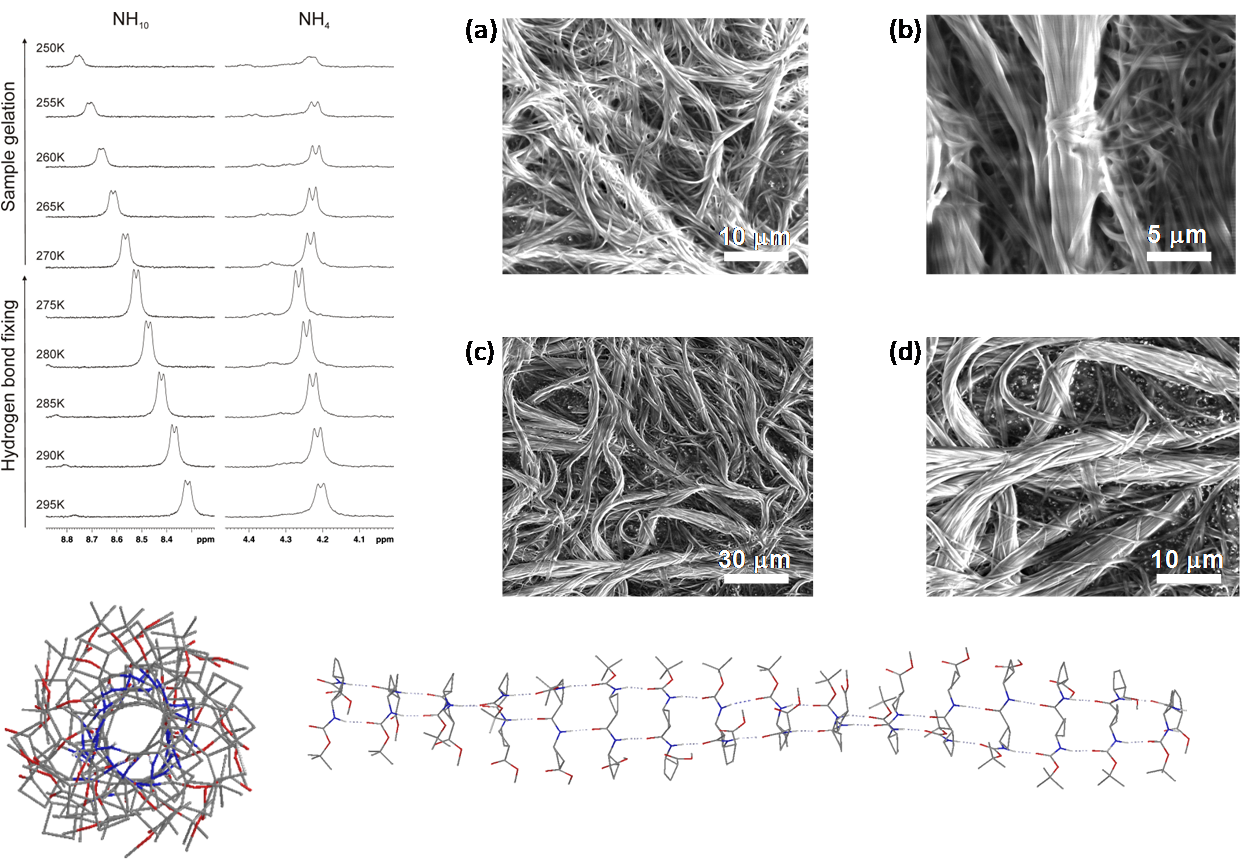
Studies on cycloalkane‐based bisamide organogelators: A new example of stochastic chiral symmetry breaking induced by sonication
Studies on cycloalkane‐based bisamide organogelators: A new example of stochastic chiral symmetry breaking induced by sonication
Ortuno, R. M., Pi-Boleda, B., Sans, M., Campos, M., Nolis, P., Illa, O., Estévez, J. C. and Branchadell, V. (2016), Chem. Eur. J.. Accepted Author Manuscript. doi:10.1002/chem.201604818
Enantiomerically pure C16-alkyl amides derived from cis and trans cycloalkane-1,2-dicarboxylic acids, respectively, have been synthesized and their behaviour as organogelators has been investigated. These compounds include cis/trans diastereomeric cyclobutane and cyclohexane derivatives with the aim to explore the influence of the ring size as well as the relative configuration in their hierarchical self-assembly to form gels. High resolution 1H NMR spectroscopy studies allowed the determination of the dynamics of the gelation process in [D8]-toluene and the sol-gel transition temperature. The morphology and size of the aggregates have been investigated and results have shown that, in the case of cyclobutane derivatives, the cis/trans stereochemistry is not relevant for the gelation behaviour and the properties of the soft-materials obtained, but it is remarkable for cyclohexane diamides, which are better organogelators. The four compounds produce chiral aggregates despite that two of them are meso achiral molecules. We show in this work that this fact is an example of stochastic symmetry breaking induced by sonication. The self-assembly of these molecules has been modelled providing information and support about the structure and the chirality of the aggregates.