J Ricardo Lucio-Gutiérrez, Paula Cordero-Pérez, Iris C Farías-Navarro, Ramiro Tijerina-Marquez, Concepción Sánchez-Martínez, José Luis Ávila-Velázquez, Pedro A García-Hernández, Homero Náñez-Terreros, Jordi Coello-Bonilla, Míriam Pérez-Trujillo, Teodor Parella, Liliana Torres-González, Noemí H Waksman-Minsky, Alma L Saucedo
Journal of Pharmaceutical and Biomedical Analysis, 2022, 19, 114885
Abstract
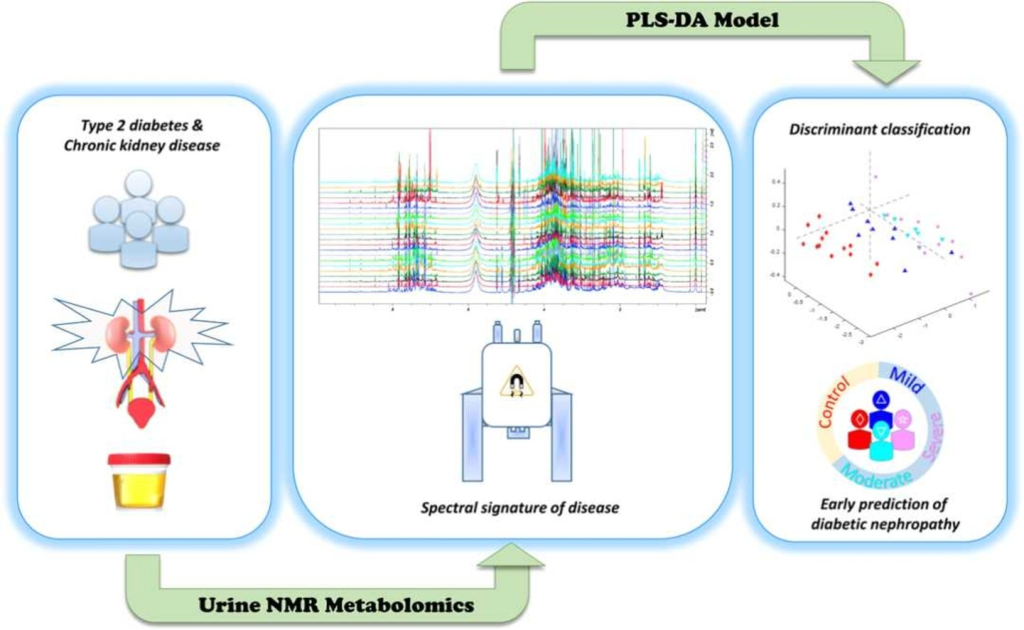
Type 2 diabetes mellitus (DM2) is a multimorbidity, long-term condition, and one of the worldwide leading causes of chronic kidney disease (CKD) –a silent disease, usually detected when non-reversible renal damage have already occurred. New strategies and more effective laboratory methods are needed for more opportune diagnosis of DM2-CKD. This study comprises clinical parameters and nuclear magnetic resonance (NMR)-based urine metabolomics data from 60 individuals (20–65 years old, 67.7% females), sorted in 5 experimental groups (healthy subjects; diabetic patients without any clinical sign of CKD; and patients with mild, moderate, and severe DM2-CKD), according to KDIGO. DM2-CKD produces a continuous variation of the urine metabolome, characterized by an increase/decrement of a group of metabolites that can be used to monitor CKD progression (trigonelline, hippurate, phenylalanine, glycolate, dimethylamine, alanine, 2-hydroxybutyrate, lactate, and citrate). NMR profiles were used to obtain a statistical model, based on partial least squares analysis (PLS-DA) to discriminate among groups. The PLS-DA model yielded good validation parameters (sensitivity, specificity, and area under the curve (AUC) of the receiver operating characteristic curve (ROC) plot: 0.692, 0.778 and 0.912, respectively) and, thus, it can differentiate between subjects with DM2-CKD in early stages, from subjects with a mild or severe condition. This metabolic signature exhibits a molecular variation associated to DM2-CKD, and data suggests it can be used to predict risk of DM2-CKD in patients without clinical signs of renal disease, offering a new alternative to current diagnosis methods.