“Simultaneous 1H and 13C NMR enantiodifferentiation from highly-resolved pure shift HSQC spectra” by Miriam Pérez-Trujillo, Laura Castañar, Eva Monteagudo, Lars T. Kuhn, Pau Nolis, Albert Virgili, R. Thomas Williamson and Teodor Parella. Chemical Communications 50:10214-10217 (2014). DOI: 10.1039/C4CC04077E
“Simultaneous 1H and 13C NMR enantiodifferentiation from highly-resolved pure shift HSQC spectra” by Miriam Pérez-Trujillo, Laura Castañar, Eva Monteagudo, Lars T. Kuhn, Pau Nolis, Albert Virgili, R. Thomas Williamson and Teodor Parella. Chemical Communications 50:10214-10217 (2014). DOI: 10.1039/C4CC04077E
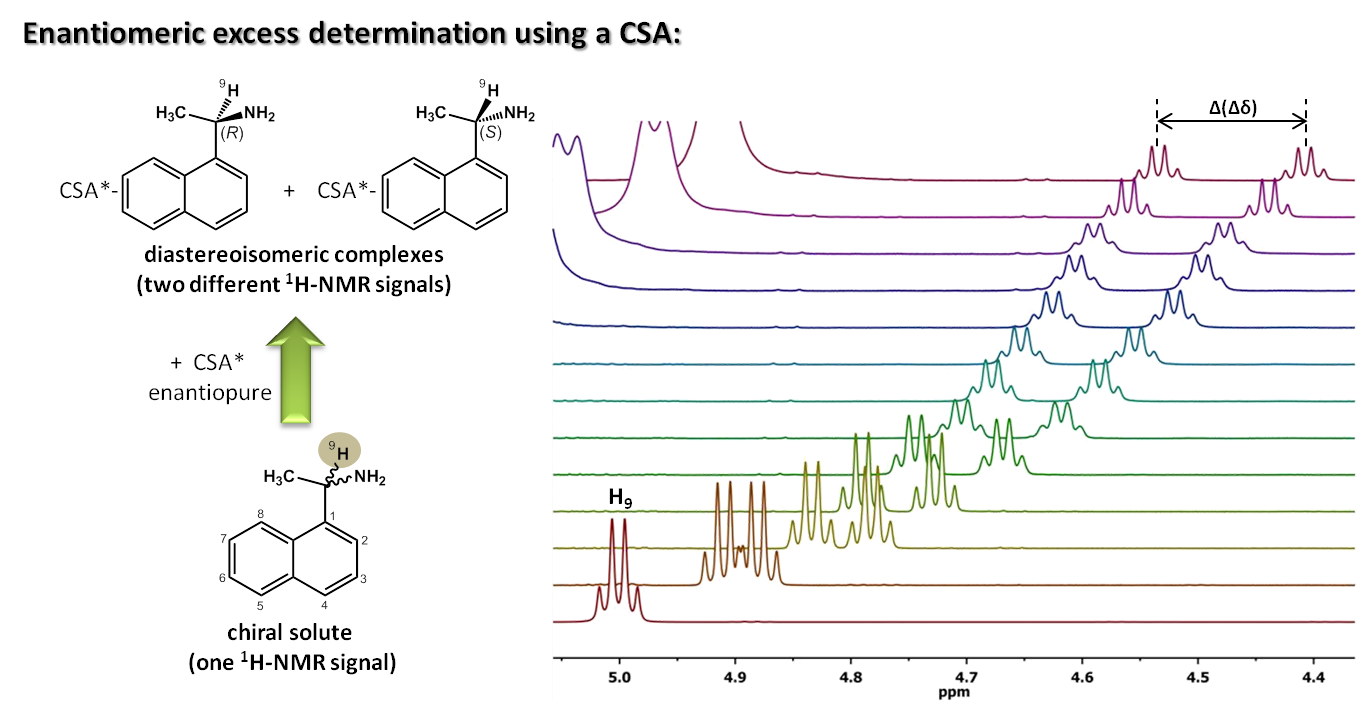
NMR-aided discrimination of enantiomers using chiral solvating agents (CSAs) is a well established method of enantiodifferentiation and measurement of enantiomeric ratios (er). The analysis is traditionally performed by observing chemical shift differences (ΔΔδ) in 1H signals by conventional 1D 1H NMR spectra. However, low ΔΔδ values and signal overlap caused by complex multiplets lead to the lack of spectral signal dispersion that preclude a straightforward analysis. Continue reading Simultaneous 1H and 13C NMR enantiodifferentiation from highly-resolved pure shift HSQC spectra

 Low-molecular-weight gelators consisting of hybrid cyclobutane-based peptides, by Sergi Celis,
Low-molecular-weight gelators consisting of hybrid cyclobutane-based peptides, by Sergi Celis,