 The relevance of the relative configuration in the folding of hybrid peptides containing β-cyclobutane amino acids and γ-amino-L-proline residues
The relevance of the relative configuration in the folding of hybrid peptides containing β-cyclobutane amino acids and γ-amino-L-proline residues
O. Illa, J.A. Olivares, P. Nolis, R.M. Ortuño
DOI: 10.1016/j.tet.2017.09.011
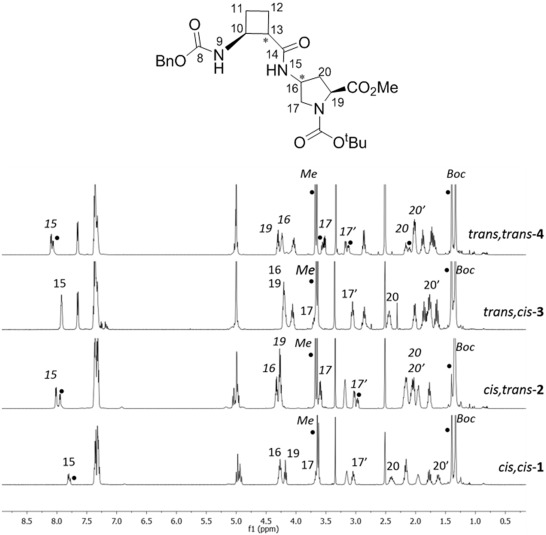
Four new series of diastereomeric β,γ-di- and β,γ-tetrapeptides derived from conveniently protected (1R,2S)- and (1S,2S)-2-aminocyclobutane-1-carboxylic acid and cis- and trans-γ-amino-l-proline joined in alternation have been synthesized. High resolution NMR experiments show that peptides containing trans-cyclobutane amino acid residues adopt a more folded structure in solution than those containing a cis-cyclobutane residue, which adopt a strand-like structure. The cis/trans relative configuration of the cyclobutane residue is the origin of the folding pattern of each peptide due to either intra- or inter-residue hydrogen-bonded ring formation, whereas the cis/trans isomerism of the γ-amino-l-proline residue does not have a significantly relevant role on the folding ability of these peptides.
 Low-molecular-weight gelators consisting of hybrid cyclobutane-based peptides, by Sergi Celis,
Low-molecular-weight gelators consisting of hybrid cyclobutane-based peptides, by Sergi Celis, 
