“Synthesis and structural study of highly constrained hybrid cyclobutane-proline γ,γ-peptides” by R. Gutiérrez-Abad, D. Carbajo, P. Nolis, C. Acosta-Silva, J. A. Cobos, O. Illa, M. Royo and R. Ortuño. Aminoacids, Volume 41, pages 673-686, 2011. DOI: 10.1007/s00726-011-0912-4.
“Synthesis and structural study of highly constrained hybrid cyclobutane-proline γ,γ-peptides” by R. Gutiérrez-Abad, D. Carbajo, P. Nolis, C. Acosta-Silva, J. A. Cobos, O. Illa, M. Royo and R. Ortuño. Aminoacids, Volume 41, pages 673-686, 2011. DOI: 10.1007/s00726-011-0912-4.
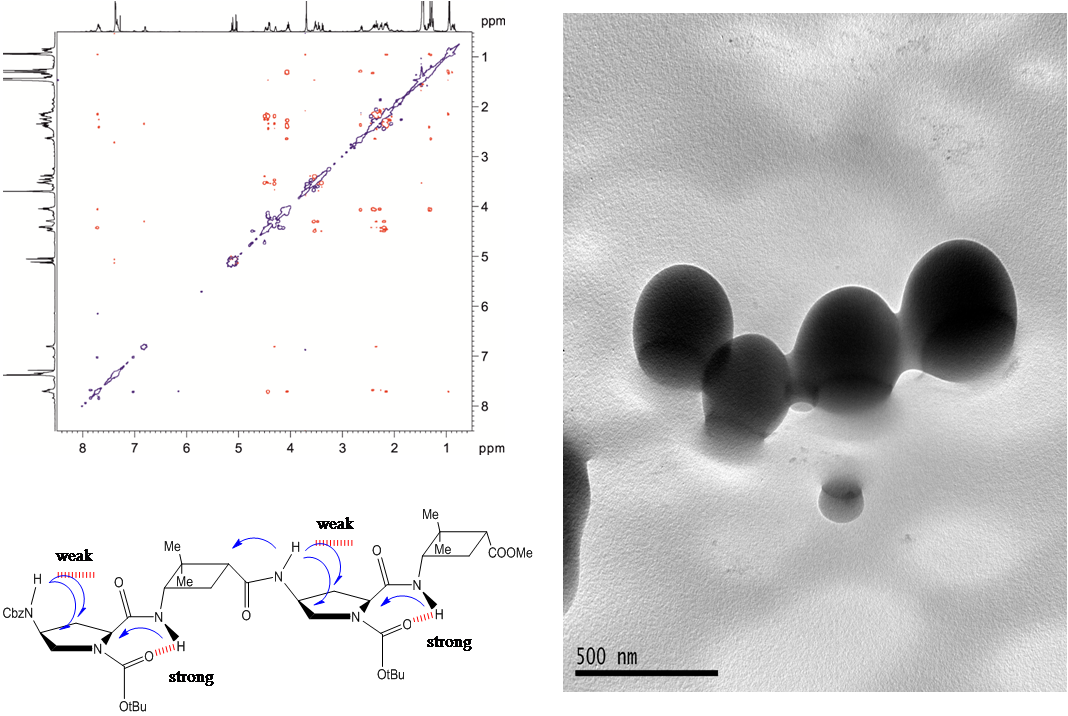
Two diastereomeric series of hybrid γ,γ-peptides derived from conveniently protected derivatives of (1R,2S)- and (1S,2R)-3-amino-2,2-dimethylcyclobutane-1-carboxylic acid and cis-4-amino-l-proline joined in alternation have efficiently been prepared through convergent synthesis. High-resolution NMR experiments show that these compounds present defined conformations in solution affording very compact structures as the result of intra and inter residue hydrogen-bonded ring formation. (R,S)-cyclobutane containing peptides adopt more twisted conformations than (S,R) diastereomers. In addition, all these γ-peptides have high tendency to aggregation providing vesicles of nanometric size, which were stable when allowed to stand for several days, as verified by transmission electron microscopy.