“Experimental evidence of Na2[B12H12] and Na formation in the desorption pathway of the 2NaBH4+ MgH2 system”, by Sebastiano Garroni, Chiara Milanese, Daphiny Pottmaier, Gabriele Mulas, Pau Nolis, Alessandro Girella, Riccarda Caputo, David Olid-Britos, Francesc Teixidor, Marcello Baricco, Amedeo Marini, Santiago Suriñach, and Maria Dolores Baró. The Journal of Physical Chemistry C, Volume 115, Pages 16664-16671, July 2011. DOI: 10.1021/jp202341j
“Experimental evidence of Na2[B12H12] and Na formation in the desorption pathway of the 2NaBH4+ MgH2 system”, by Sebastiano Garroni, Chiara Milanese, Daphiny Pottmaier, Gabriele Mulas, Pau Nolis, Alessandro Girella, Riccarda Caputo, David Olid-Britos, Francesc Teixidor, Marcello Baricco, Amedeo Marini, Santiago Suriñach, and Maria Dolores Baró. The Journal of Physical Chemistry C, Volume 115, Pages 16664-16671, July 2011. DOI: 10.1021/jp202341j
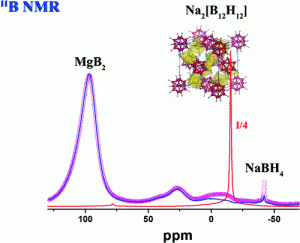
In the present work we report the desorption pathway of the 2NaBH4 + MgH2 system. Ex-situ X-ray powder diffraction (XRPD) and solid state magic angle spinning (MAS) nuclear magnetic resonance (NMR) measurements have been performed on samples heat-treated up to 450°C for different times. Ex-situ X-ray powder diffraction experiments conducted on fully desorbed samples allowed to identify nanocrystalline MgB2 and metallic Na as dehydrogenation products. 11B and 23Na NMR analyses have been also carried out in order to evaluate the structural evolution of decomposed materials. Our measurements show that the local structure of MgB2 is influenced by the replacement of Mg with Na atoms in the Mg sites. Moreover, amorphous Na2[B12H12] was detected in the partially desorbed sample and in the final products of the decomposition reaction. The presence of the [B12H12]2- anion was confirmed by both direct comparison with the 11B{1H} NMR spectrum of pure Na2[B12H12] and dynamic Cross Polarization experiments.