 “Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies” by Reyes B, Coca MI, Codinach M, López-Lucas MD, Del Mazo-Barbara A, Caminal M, Oliver-Vila I, Cabañas V, S. Lope-Piedrafita, García-López J, Moraleda JM, Fontecha CG, Vives J. Cytotherapy. 2017 :1060-1069. doi: 10.1016/j.jcyt.2017.06.004.
“Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies” by Reyes B, Coca MI, Codinach M, López-Lucas MD, Del Mazo-Barbara A, Caminal M, Oliver-Vila I, Cabañas V, S. Lope-Piedrafita, García-López J, Moraleda JM, Fontecha CG, Vives J. Cytotherapy. 2017 :1060-1069. doi: 10.1016/j.jcyt.2017.06.004.
Biodistribution of candidate cell-based therapeutics is a critical safety concern that must be addressed in the preclinical development program. We aimed to design a decision tree based on a series of studies included in actual dossiers approved by competent regulatory authorities, noting that the design, execution and interpretation of pharmacokinetics studies using this type of therapy is not straightforward and presents a challenge for both developers and regulators. This work contributes to the standardization in the design of biodistribution studies by improving methods for accurate assessment of safety.
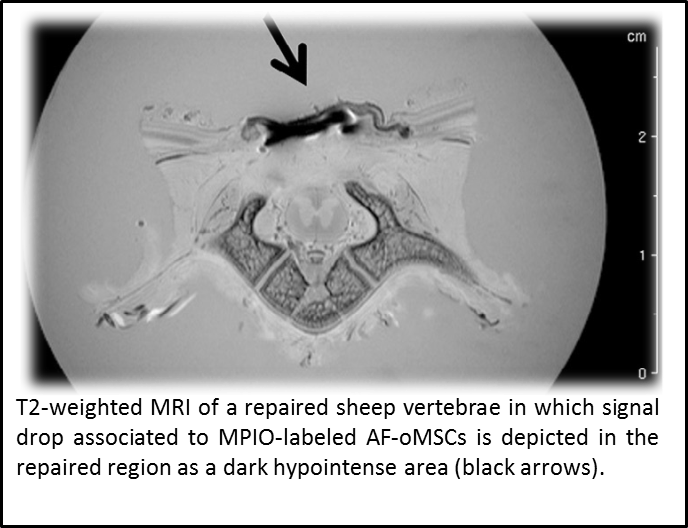
Eight studies were evaluated for the definition of a decision tree, in which mesenchymal stromal cells (MSCs) were administered to mouse, rat and sheep models using diverse routes (local or systemic), cell labeling (chemical or genetic) and detection methodologies (polymerase chain reaction (PCR), immunohistochemistry (IHC), fluorescence bioimaging, and magnetic resonance imaging (MRI). Moreover, labeling and detection methodologies were compared in terms of cost, throughput, speed, sensitivity and specificity.