“The Role of the Chiral cis-1,3-Disubstituted 2,2-Dimethylcyclobutane Motif in the Conformational Bias of Several Types of γ-Peptides” by Jordi Aguilera, Juan A. Cobos, Raquel Gutiérrez-Abad, Carles Acosta, Pau Nolis, Ona Illa, Rosa M. Ortuño. Eur. J. Org. Chem. 2013 (early view). DOI: 10.1002/ejoc.201300066
“The Role of the Chiral cis-1,3-Disubstituted 2,2-Dimethylcyclobutane Motif in the Conformational Bias of Several Types of γ-Peptides” by Jordi Aguilera, Juan A. Cobos, Raquel Gutiérrez-Abad, Carles Acosta, Pau Nolis, Ona Illa, Rosa M. Ortuño. Eur. J. Org. Chem. 2013 (early view). DOI: 10.1002/ejoc.201300066
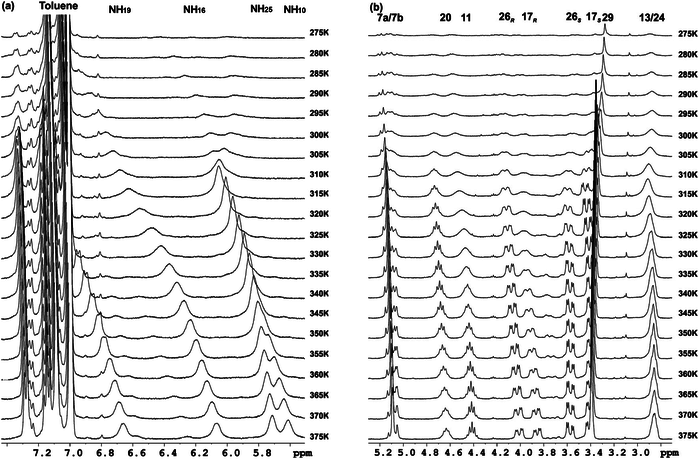
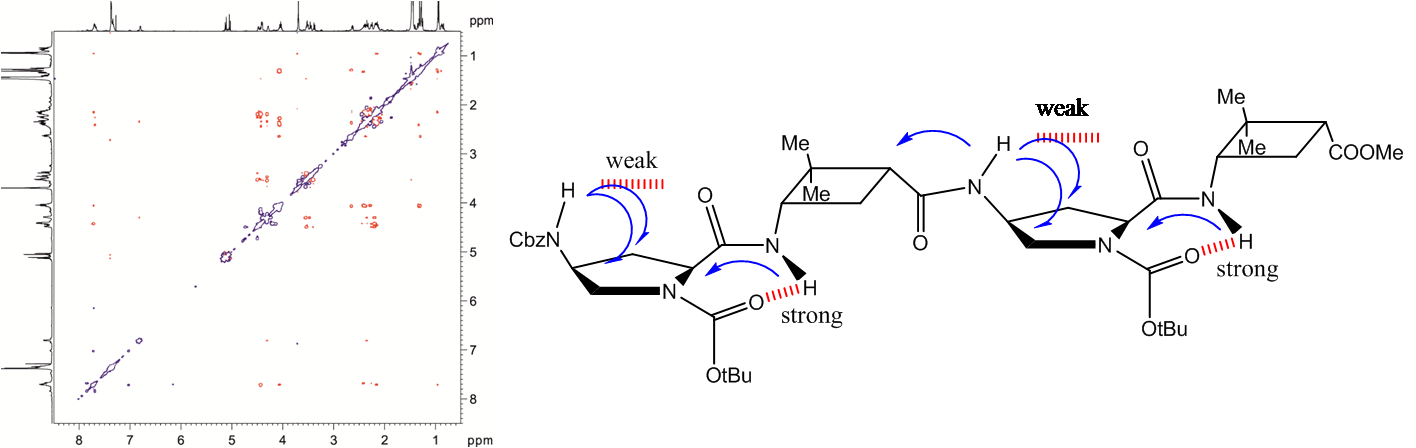
Three series of new γ-peptides have been synthesized by starting from conveniently protected cis-3-amino-2,2-dimethylcyclobutane-1-carboxylic acid derivatives. The first series is constructed with only one enantiomer of this γ-amino acid, whereas in the second one both enantiomeric cyclobutane residues are joined in alternating fashion. The high degrees of rigidity in these γ-peptides induce the adoption of extended but sterically constrained conformations in both cases. The third series is the product of alternation of a cyclobutane residue with linear γ-aminobutyric acid (GABA). Conformational bias in these three systems accounts for the cyclobutane being a major disrupting factor to the formation of strong intramolecular hydrogen bonds, leading to extended conformations. In contrast, investigation of cyclobutane/GABA hybrid γ-peptides of a fourth series, in which the carbocyclic moiety is not a part of the polyamide skeleton but acts as a chiral polyfunctional platform, reveals that these peptides are able to produce intramolecular hydrogen bonds leading to well defined folded conformations.