Stereoselectivity of Proline / Cyclobutane Amino Acid-Containing Peptide Organocatalysts for Asymmetric Aldol Additions: a Rationale
Stereoselectivity of Proline / Cyclobutane Amino Acid-Containing Peptide Organocatalysts for Asymmetric Aldol Additions: a Rationale
J. Org. Chem., Just Accepted Manuscript
DOI: 10.1021/acs.joc.7b02745
Publication Date (Web): November 29, 2017
Abstract
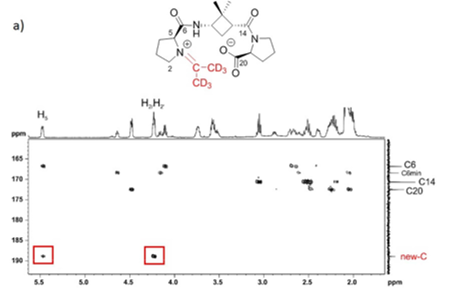
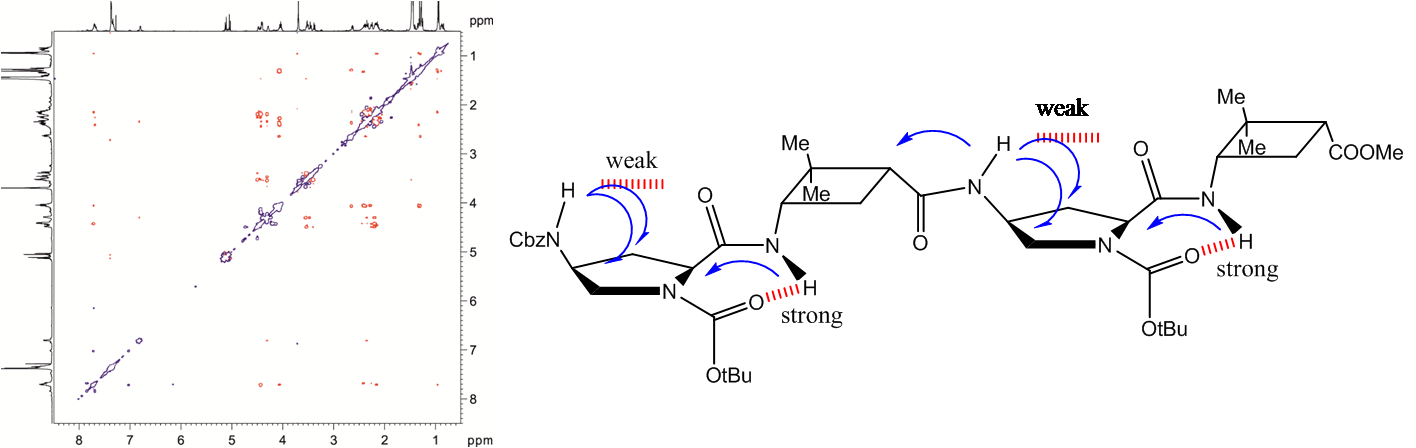
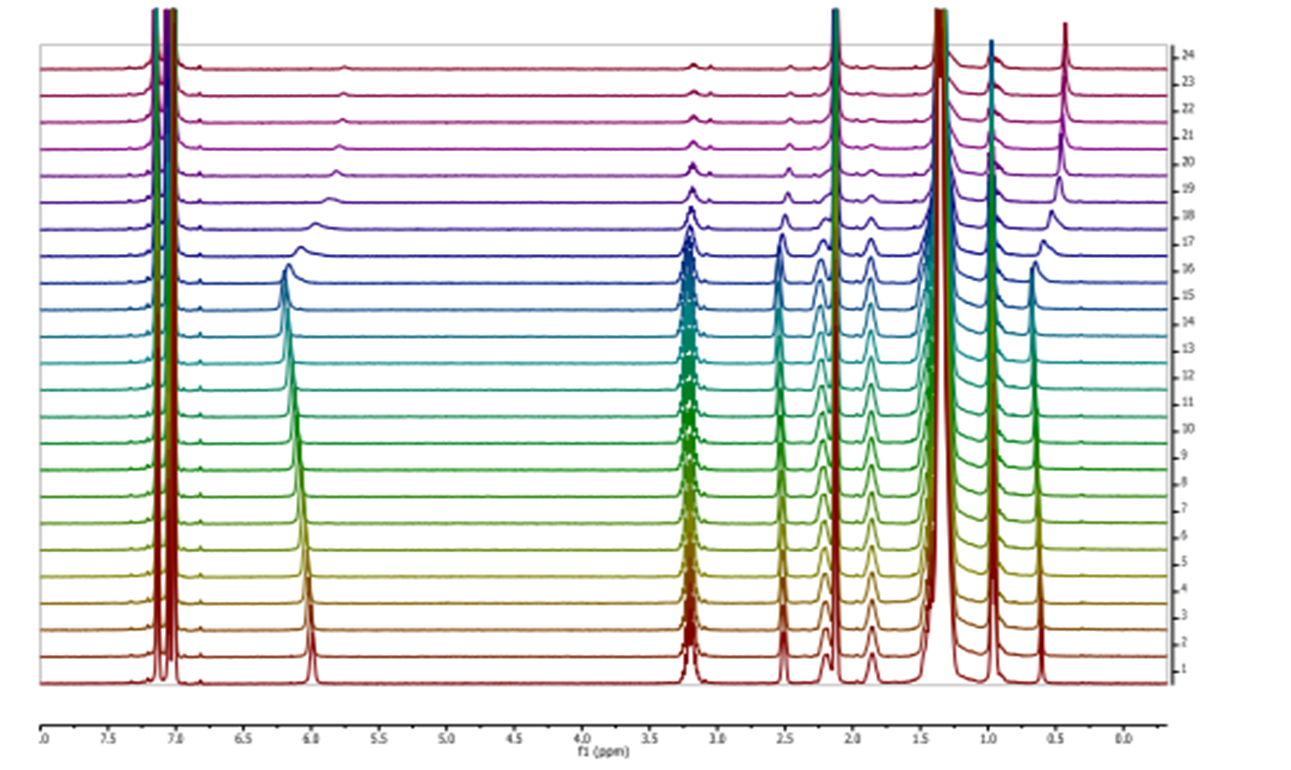
Several α,β,α- or α,γ,α-tripeptides, consisting of a central cyclobutane β- or γ-amino acid being flanked by two (D)- or (L)-proline residues, have been synthesized and tested as organocatalysts in asymmetric aldol additions. High yields and enantioselectivities have been achieved with α,γ,α-tripeptides, being superior to the peptides containing a cyclobutane β-amino acid residue. This can probably be due to their high rigidity, which hinders the peptide catalysts to adopt the proper active conformation. This reasoning correlates with the major conformation of the peptides in the ground state, as suggested by 1H NMR and computational calculations. The configuration of the aldol products is controlled by the proline chirality, and consequently, the R/S configuration of aldol products can be tuned by the use of either commercially available (D)- or (L)-proline enantiomers. The enantioselectivity in the aldol reactions is reversed if the reactions are carried out in the presence of water or other protic solvents such as methanol. Spectroscopic and theoretical investigations revealed that this effect is not the consequence of conformational changes in the catalyst but rather caused by the participation of a water molecule in the rate determining transition state, in such a way that the preferential nucleophilic attack is oriented to the opposite enantiotopic aldehyde face.
Related Posts