31P-NMR Metabolomics Revealed Species-Specific Use of Phosphorous in Trees of a French Guiana Rainforest, by Gargallo-Garriga, A.; Sardans, J.; Llusià, J.; Peguero, G.; Asensio, D.; Ogaya, R.; Urbina, I.; Langenhove, L.V.; Verryckt, L.T.; Courtois, E.A.; Stahl, C.; Grau, O.; Urban, O.; Janssens, I.A.; Nolis, P.; Pérez-Trujillo, M.; Parella, T.; Peñuelas, J. Molecules 2020, 25, 3960. https://doi.org/10.3390/molecules25173960
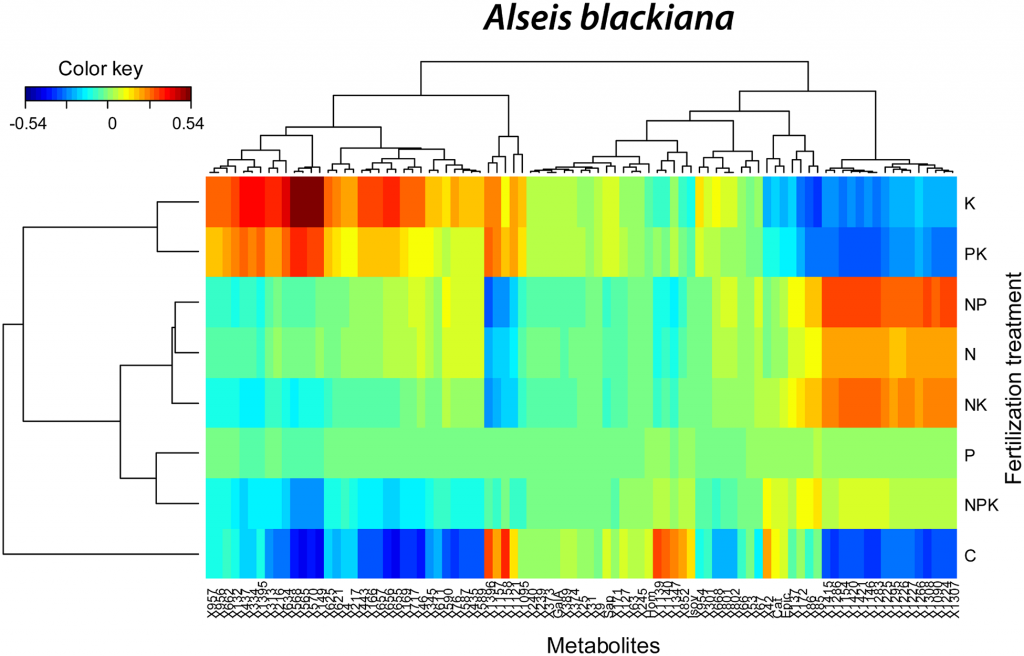
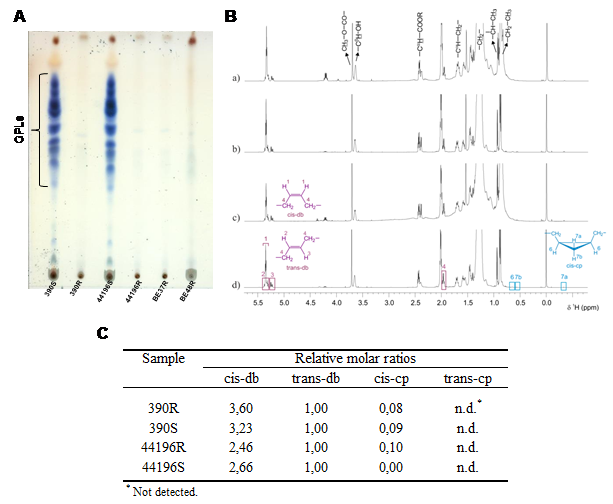
Productivity of tropical lowland moist forests is often limited by availability and functional allocation of phosphorus (P) that drives competition among tree species and becomes a key factor in determining forestall community diversity. We used non-target 31P-NMR metabolic profiling to study the foliar P-metabolism of trees of a French Guiana rainforest. The objective was to test the hypotheses that P-use is species-specific, and that species diversity relates to species P-use and concentrations of P-containing compounds, including inorganic phosphates, orthophosphate monoesters and diesters, phosphonates and organic polyphosphates. We found that tree species explained the 59% of variance in 31P-NMR metabolite profiling of leaves. A principal component analysis showed that tree species were separated along PC 1 and PC 2 of detected P-containing compounds, which represented a continuum going from high concentrations of metabolites related to non-active P and P-storage, low total P concentrations and high N:P ratios, to high concentrations of P-containing metabolites related to energy and anabolic metabolism, high total P concentrations and low N:P ratios. These results highlight the species-specific use of P and the existence of species-specific P-use niches that are driven by the distinct species-specific position in a continuum in the P-allocation from P-storage compounds to P-containing molecules related to energy and anabolic metabolism.
This article belongs to the Special Issue:
https://www.mdpi.com/journal/molecules/special_issues/nmr_metabolomics