Some of our recent research work was presented at the European NMR meeting Euromar 2021 that was going to take place at Portoroz (Slovenia), but which was finally virtual from the 5th to the 8th of July 2021.
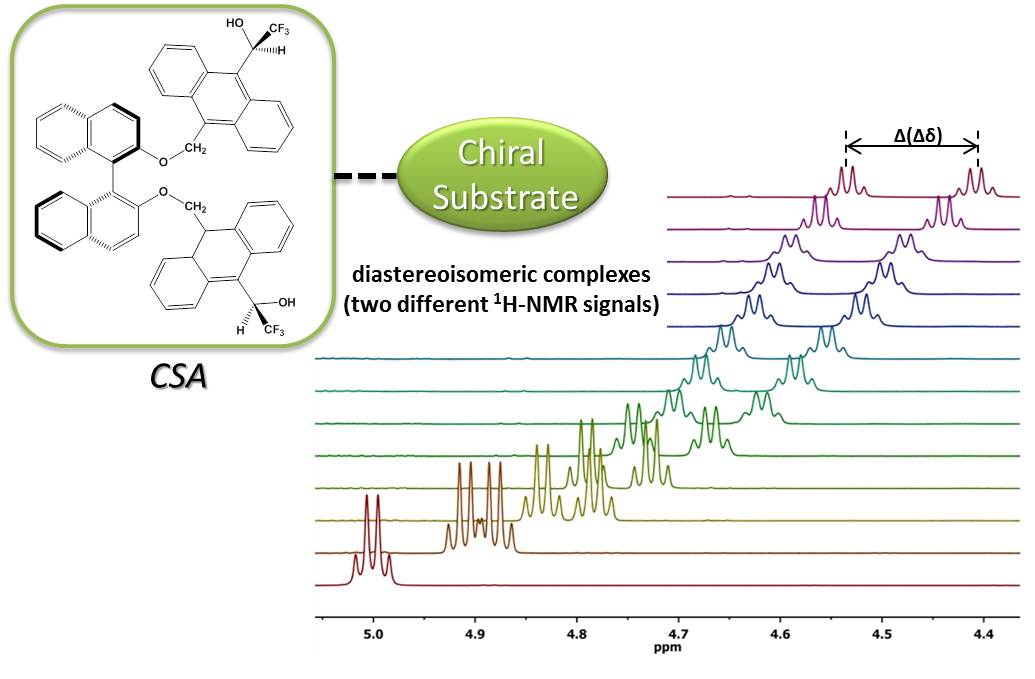
· Míriam Pérez-Trujillo presented the talk In situ Enantiospecific Detection of Multiple Metabolites in Mixtures using NMR Spectroscopy in the “Metabolomics” session. In this talk our last advances in enantiodifferentiation using NMR were shown and discussed.
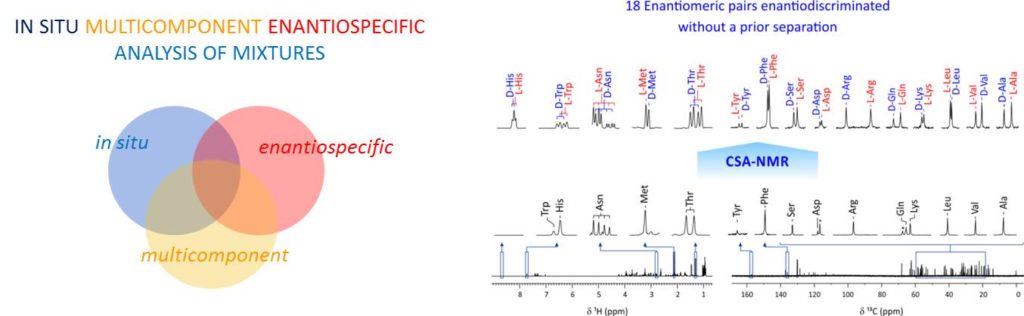
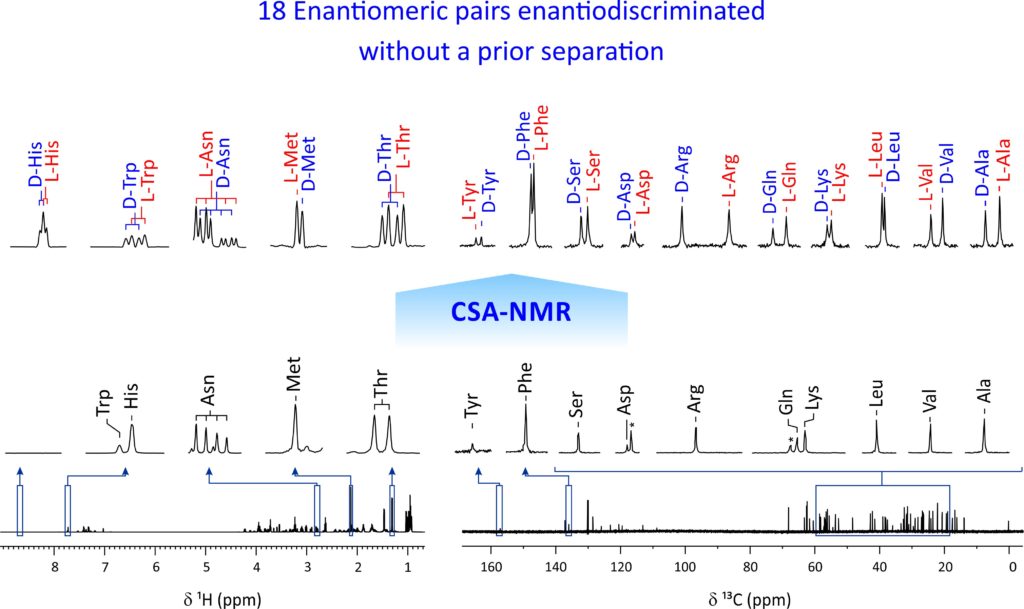
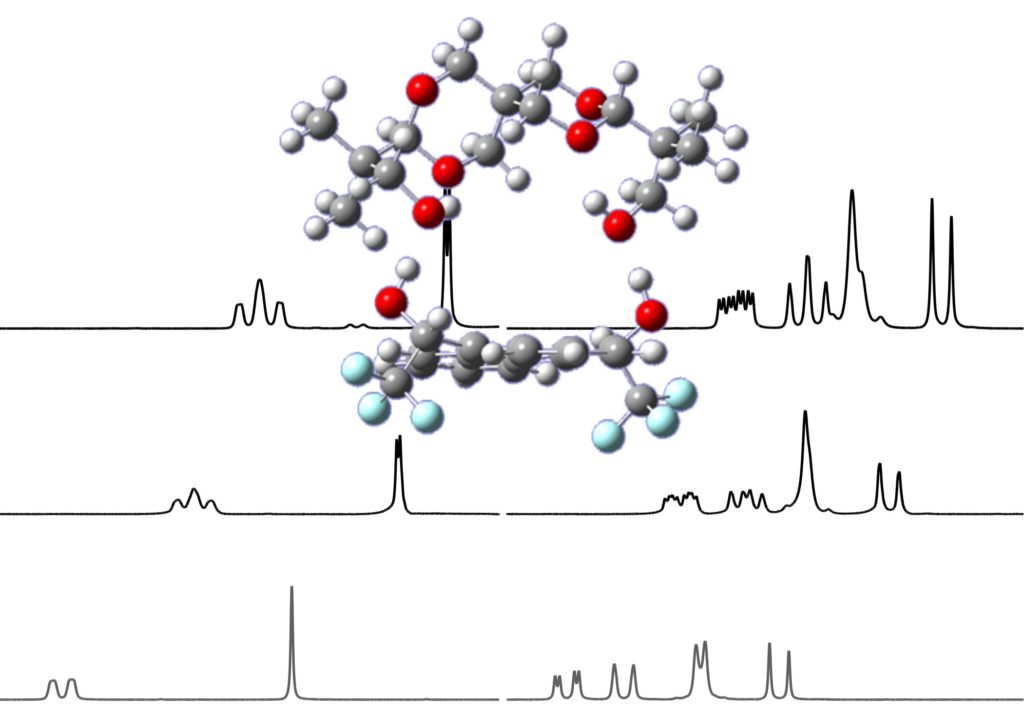
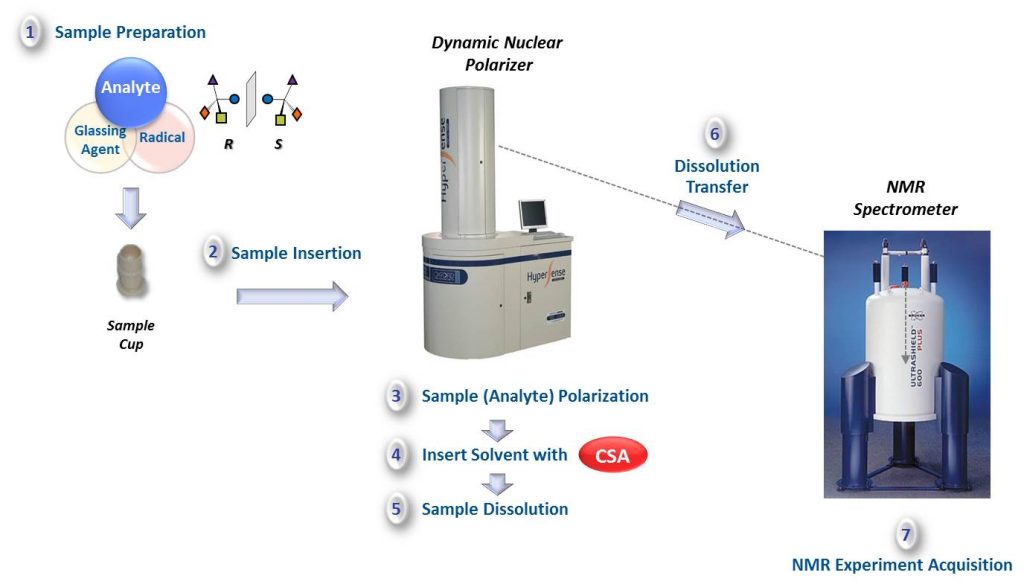
To date, the enantiospecific analysis of mixtures necessarily requires prior separation of the individual components. The simultaneous enantiospecific detection of multiple chiral molecules in a mixture represents a major challenge, which would lead to a significantly better understanding of the underlying biological processes; e.g. via enantiospecifically analyzing metabolites in their native environment. Here, we report on the first in situ enantiospecific detection of a thirty-nine-component mixture. As a proof of concept, eighteen essential amino acids (AAs) at physiological concentrations were simultaneously enantiospecifically detected using NMR spectroscopy and a chiral solvating agent. This work represents a first step towards the simultaneous multicomponent enantiospecific analysis of complex mixtures, a capability that will have substantial impact on metabolism studies, metabolic phenotyping, chemical reaction monitoring, and many other fields where complex mixtures containing chiral molecules require efficient characterization.