“Role of aluminum chloride on the reversible hydrogen storage properties of the Li-N-H system”
“Role of aluminum chloride on the reversible hydrogen storage properties of the Li-N-H system”
Fernández, L.; Garroni, S.; Larochette, P.; Nolis, P.; Mulas, G.; Enzo, S.; Baró, M.D.; Gennari, F. International Journal of Hydrogen Energy, IN PRESS 2015 doi:10.1016/j.ijhydene.2015.08.030
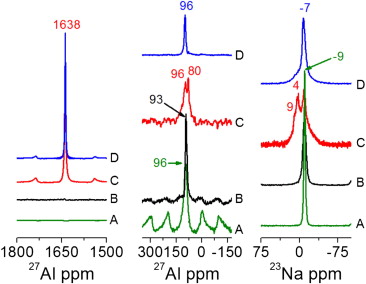
In order to understand the role of AlCl3 addition on the Li–N–H system, we have systematically investigated the hydrogen sorption kinetics and the reactions between LiNH2–LiH and AlCl3 additive with a multitechnique approach involving differential scanning calorimetry (DSC), hydrogen volumetric measurements, X-ray powder diffraction (XRPD), Fourier transform infrared analysis (FTIR) and solid-state nuclear magnetic resonance (NMR). Continue reading Storage properties of the Li-N-H system