Lucas Bragança Carvalho, Pricila Maria BatistaChagas, Tamara Rezende Marques, Angelina Razafitianamaharavo, Manuel Pelletier, Pau Nolis, Carlos Jaime, Sérgio Scherrer Thomasi, Lucianade Matos Alves Pinto.
Journal of Environmental Chemical Engineering – Available online 20 October 2019
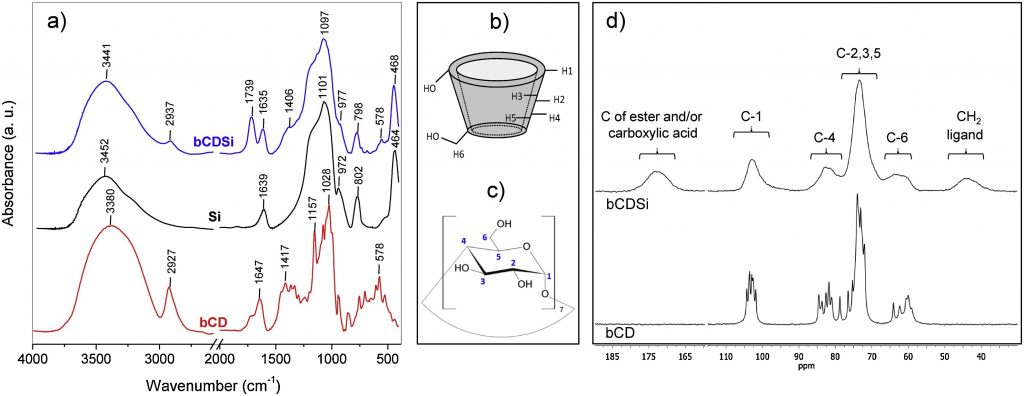
ABSTRACT Contamination of water with steroid residues can cause a number of environmental damages, affecting exposed organisms including man. The development of technologies for treatment or removal of this type of micropollutant from water is of paramount importance. In this study, citric acid was used to functionalize β-cyclodextrin (bCD) on the silica surface generating an organic-inorganic hybrid composite for application in molecular sequestration. The functionalization percentage was high, with about 62.6% of the composite mass corresponding to the organic part of the material. 13C NMR and infrared spectroscopic analysis indicate that the functionalization mechanism occurs by an esterification reaction between the citric acid with the silanol groups from silica and the primary hydroxyls of the bCDs. Fast adsorption of the methyltestosterone steroid was observed at acid pH, with a high adsorption capacity of 11 mg g-1. The best kinetic and isotherm models fit indicated that the adsorption occurred by a physical mechanism at independent sites with the steroid molecule possibly captured by two bCDs. The removal process was spontaneous and exothermic, with the existence of weak interactions between the hormone and the composite, and its regeneration is quite fast efficient with the displacement of the complexation equilibrium. The results obtained in this study demonstrate the considerable potential of the composite for use in the treatment of wastewater containing the steroid studied, and its efficacy should be evaluated for other steroid molecules.