“Designing hybrid foldamers: The effect on the peptide conformational bias of beta- versus alpha- and gamma-linear residues in alternation with (1R,2S)-2-aminocyclobutane-1-carboxylic acid” Sergi Celis, Esther Gorrea, Pau Nolis, Ona Illa, Rosa Maria Ortuño. Organic and Biomolecular Chemistry. Volume 10, Pages 861-868, October 2011 DOI: 10.1039/C1OB06575K
“Designing hybrid foldamers: The effect on the peptide conformational bias of beta- versus alpha- and gamma-linear residues in alternation with (1R,2S)-2-aminocyclobutane-1-carboxylic acid” Sergi Celis, Esther Gorrea, Pau Nolis, Ona Illa, Rosa Maria Ortuño. Organic and Biomolecular Chemistry. Volume 10, Pages 861-868, October 2011 DOI: 10.1039/C1OB06575K
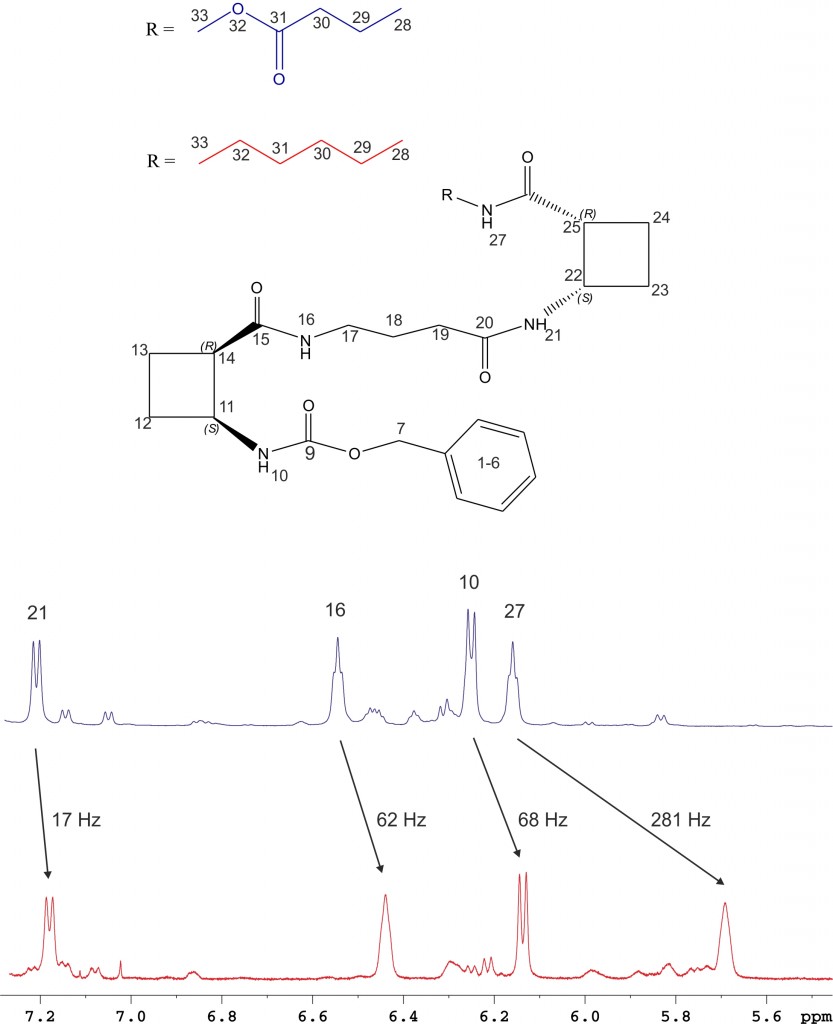
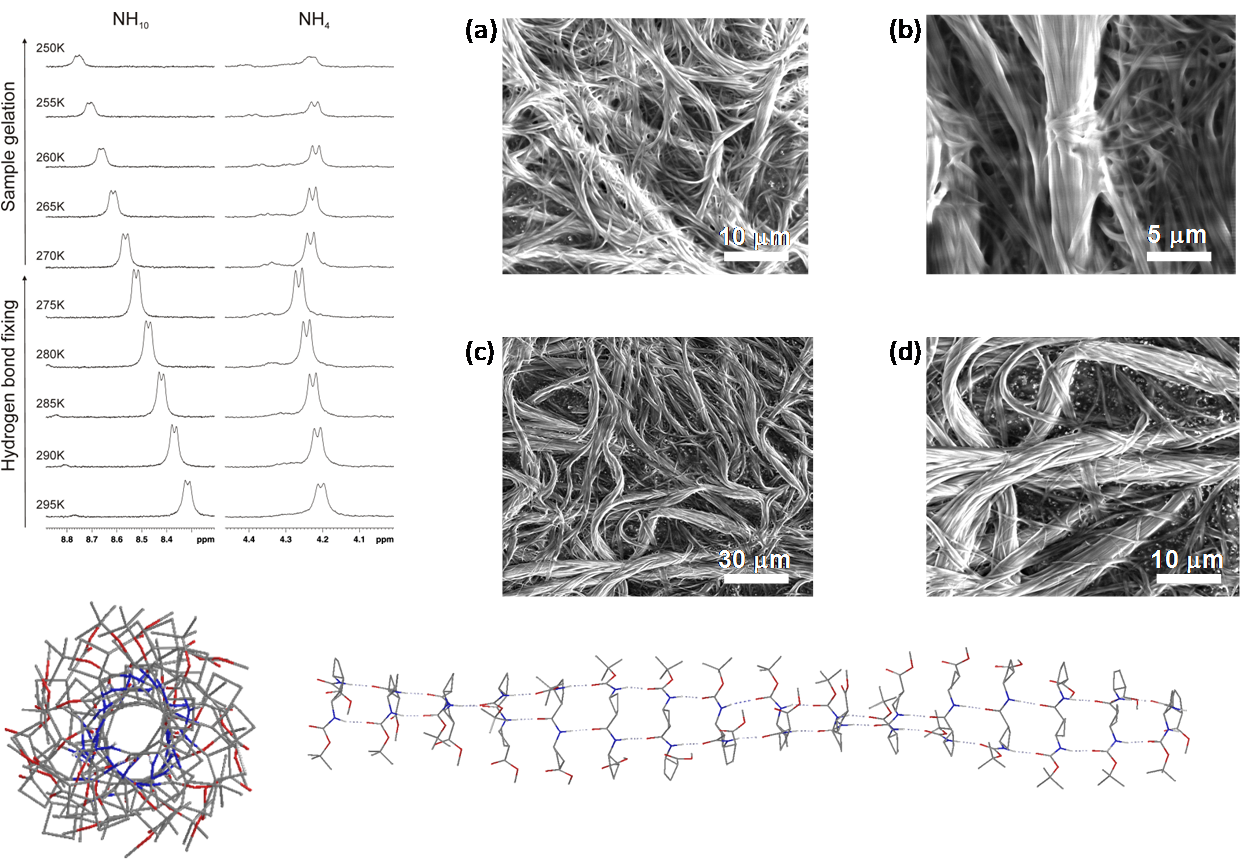
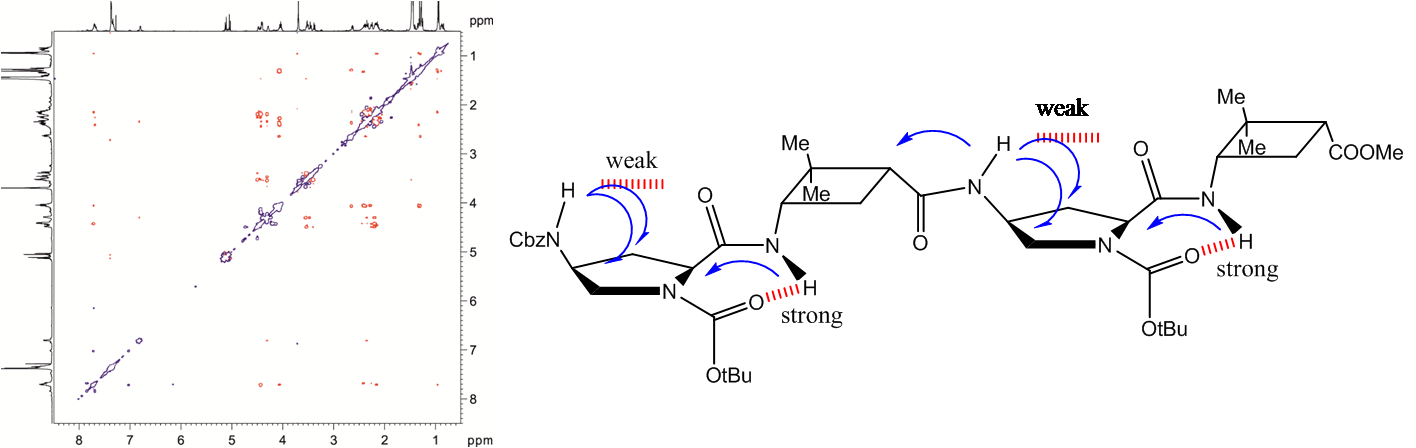
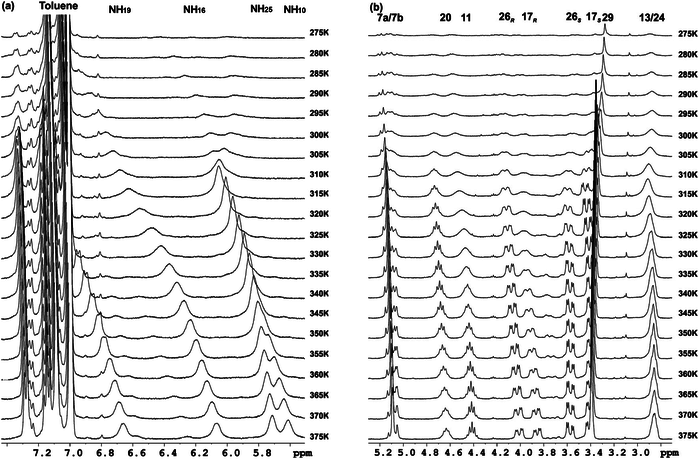
Several oligomers constructed with (1R,2S)-2-aminocyclobutane-1-carboxylic acid and glycine, beta-alanine, and gamma-amino butyric acid (GABA), respectively, joined in alternation have been synthesized and studied by means of NMR and CD experiments as well as with computational calculations. Results account for the spacer length effect on folding and show that conformational preference for these hybrid peptides can be tuned from beta-sheet-like folding for those containing a C2 or C4 linear segment to a helical folding for those with a C3 spacer between cyclobutane residues. The introduction of cyclic spacers between these residues does not modify the extended ribbon-type structure previously manifested in poly(cis-cyclobutane) beta-oligomers.