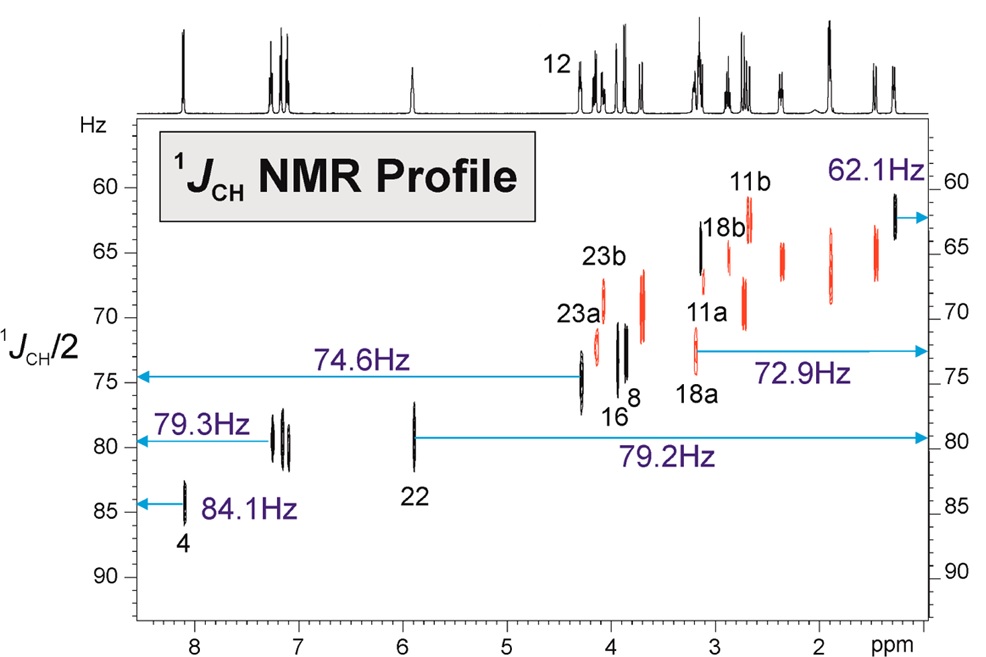
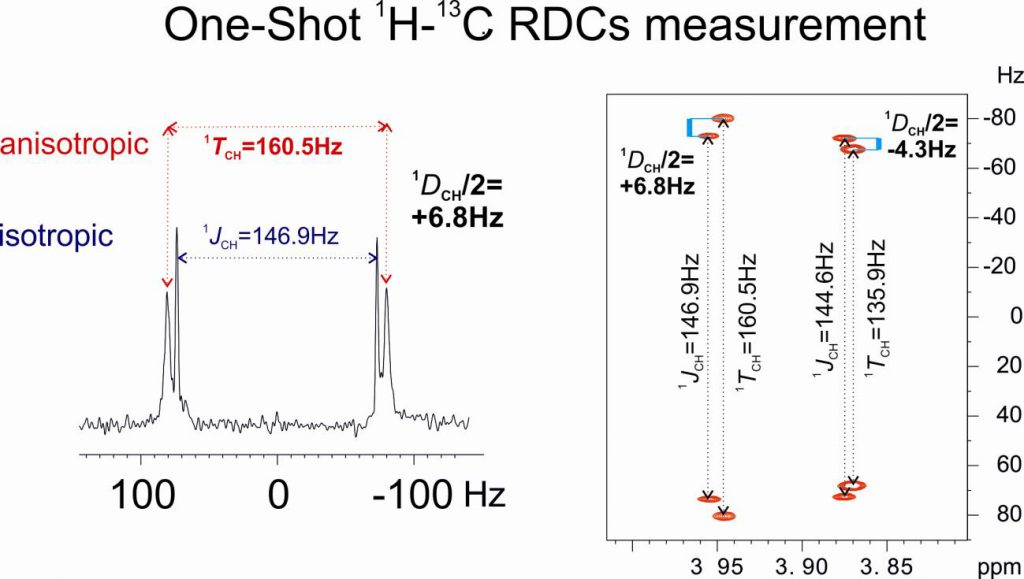
“ 1JCH NMR Profile: Identification of key structural features and functionalities by visual observation and direct measurement of one-bond proton-carbon coupling constants” by Núria Marcó, A.A. Souza, Pau Nolis, Carlos Cobas, R. R. Gil and Teodor Parella. Journal of Organic Chemistry 2017, 276 : 37.42. DOI: 10.1021/acs.joc.6b02873
A user-friendly NMR interface for the visual and accurate determination of experimental one-bond proton-carbon coupling constants (1JCH) in small molecules is presented. This intuitive 1JCH profile correlates directly delta(1H) and 1JCH facilitates the rapid identification and assignment of 1H signals belonging to key structural elements and functional groups. Illustrative examples are provided for some target molecules including terminal alkynes, strained rings, electronegative substituents or lone-pair bearing heteronuclei.