“Structural discrimination from in-situ measurement of 1DCH and 2DHH RDCs” by Núria Marcó, R. R. Gil and Teodor Parella. Magnetic Resonance in Chemistry 2017, DOI:
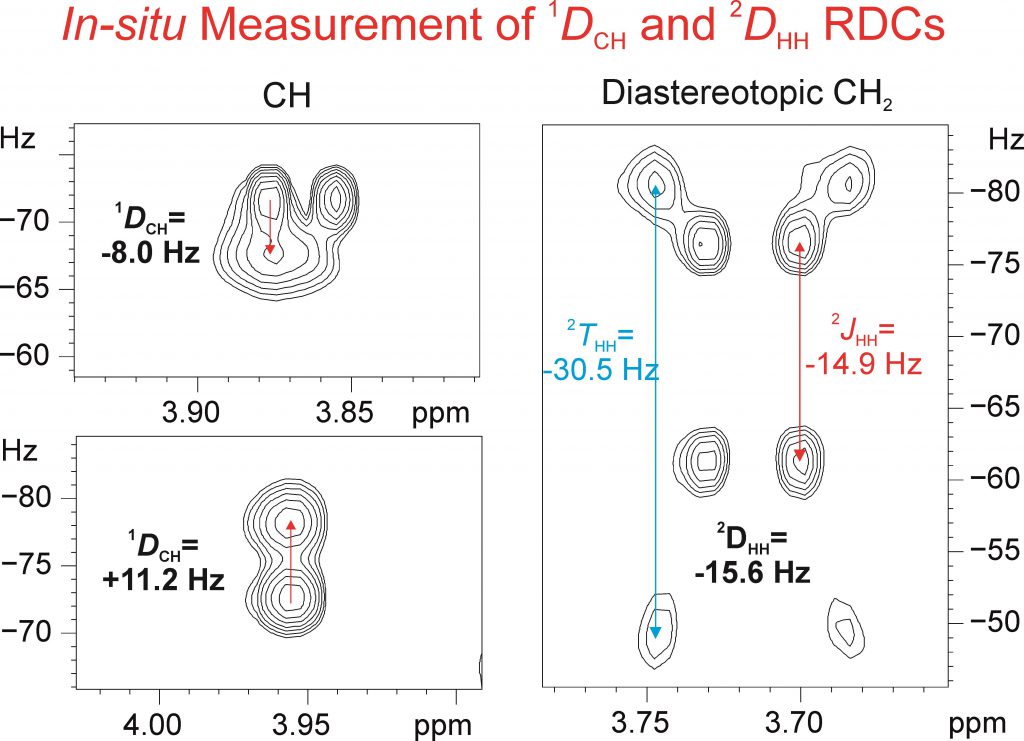
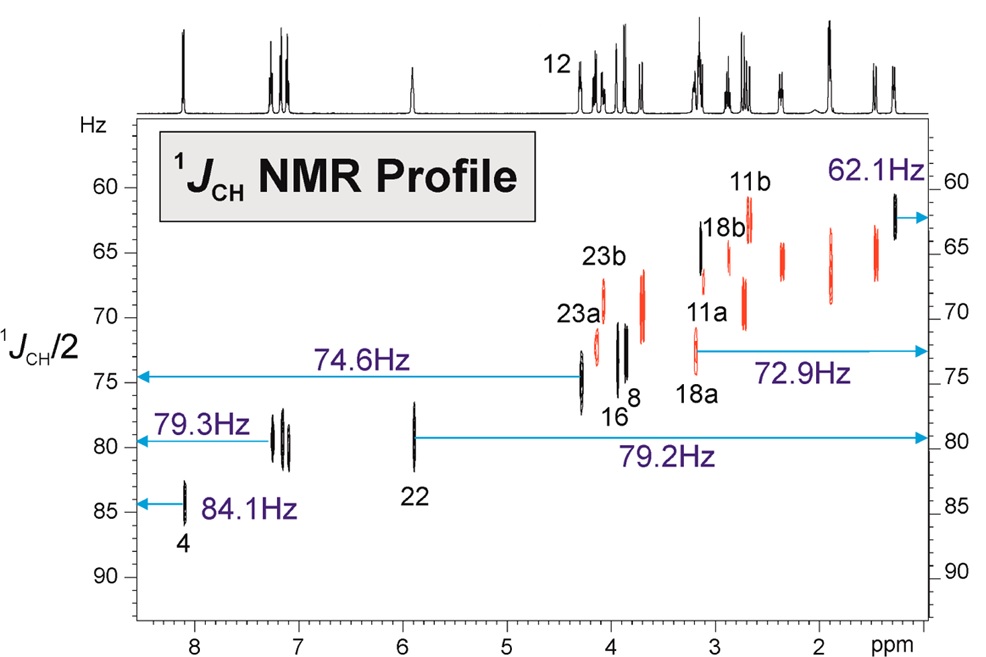
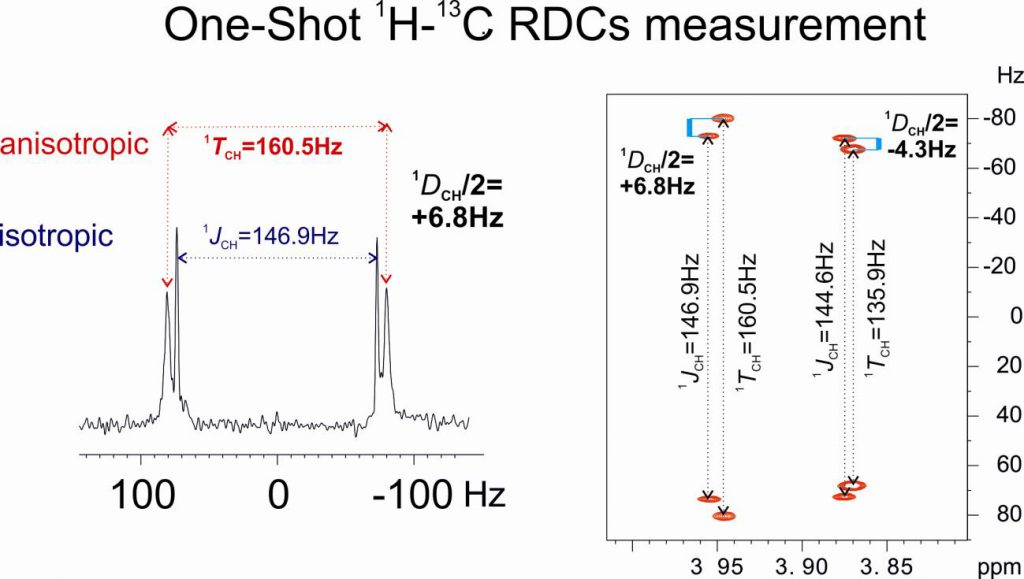
A fast RDC-assisted strategy involving the simultaneous determination of scalar and total coupling constants from a single 1JCH/2JHH-resolved NMR spectrum is reported. It is shown that the concerted use of the directly measured 1DCH (for all CHn multiplicities) and 2DHH residual dipolar couplings allows an on-the-fly assignment of diastereotopic CH2 protons, as well as of an efficient discrimination between all eight possible diastereoisomeric structures of strychnine, which contains six stereocenters.
Pulse Programs Code for Bruker:
Data set Example: