“Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez, S. Lope-Piedrafita, E. Martínez-Membrives, R. Torrubia, A. Tobeña, and A. Fernández-Teruel. European Neuropsychopharmacology, 2017, 27: 146–158. DOI: 10.1016/j.euroneuro.2016.12.003
“Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez, S. Lope-Piedrafita, E. Martínez-Membrives, R. Torrubia, A. Tobeña, and A. Fernández-Teruel. European Neuropsychopharmacology, 2017, 27: 146–158. DOI: 10.1016/j.euroneuro.2016.12.003
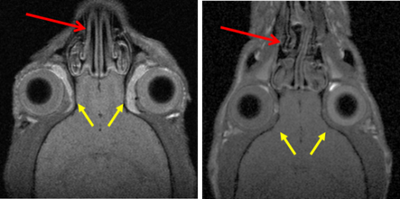
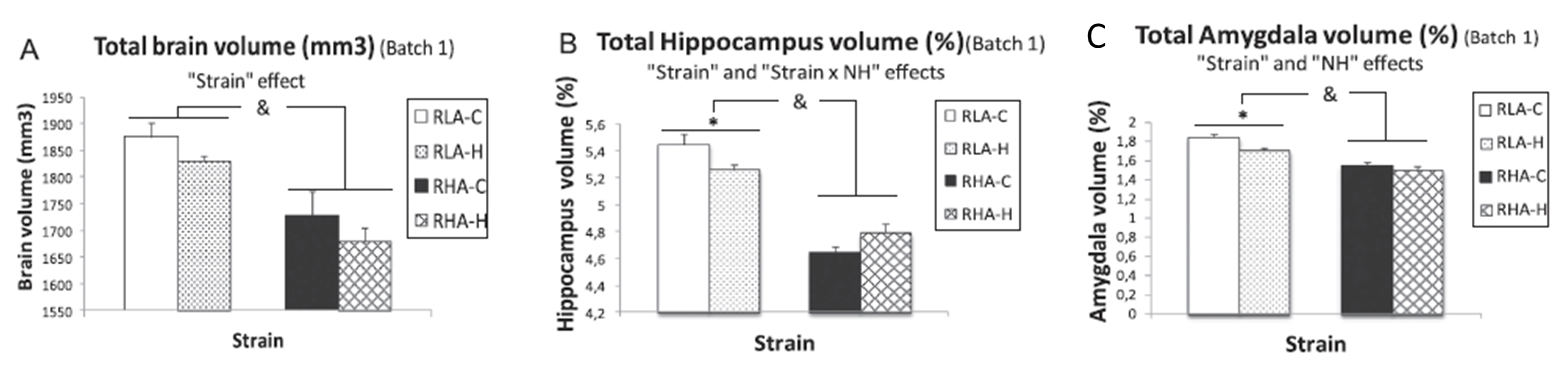
The hippocampus and amygdala have been proposed as key neural structures related to anxiety. A more active hippocampus/amygdala system has been related to greater anxious responses in situations involving conflict/novelty. The Roman Low- (RLA) and High-avoidance (RHA) rat strains constitute a genetic model of differential anxiety. Relative to RHA rats, RLA rats exhibit enhanced anxiety/fearfulness, augmented hippocampal/amygdala c-Fos expression following exposure to novelty/conflict, increased hippocampal neuronal density and higher endocrine responses to stress. Neonatal handling (NH) is an environmental treatment with long-lasting anxiety/stress-reducing effects in rodents. Since hippocampus and amygdala volume are supposed to be related to anxiety/fear, it was hypothesized a greater volume of both areas in RLA than in RHA rats, as well as that NH treatment would reduce anxiety and the volume of both structures. Adult untreated and NH-treated RHA and RLA rats were tested for anxiety, sensorimotor gating (PPI), stress-induced corticosterone and prolactin responses, two-way active avoidance acquisition and in vivo 7 T 1H-Magnetic resonance image.
As expected, untreated RLA rats showed higher anxiety and post-stress hormone responses, as well as greater hippocampus and amygdala volumes than untreated RHA rats. NH decreased anxiety/stress responses, especially in RLA rats, and significantly reduced hippocampus and amygdala volumes in this strain. Dorsal striatum volume was not different between the strains nor it was affected by NH. Finally, there were positive associations (as shown by correlations, factor analysis and multiple regression) between anxiety and PPI and hippocampus/amygdala volumes.

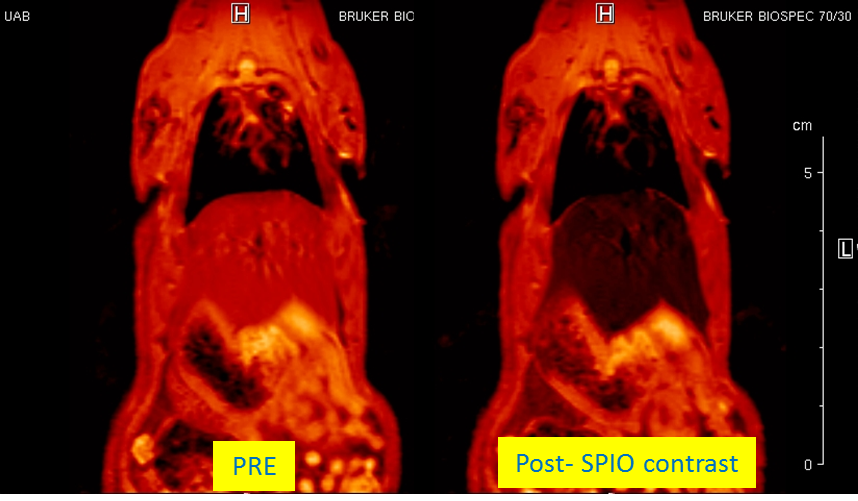

 “Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies” by Reyes B, Coca MI, Codinach M, López-Lucas MD, Del Mazo-Barbara A, Caminal M, Oliver-Vila I, Cabañas V,
“Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies” by Reyes B, Coca MI, Codinach M, López-Lucas MD, Del Mazo-Barbara A, Caminal M, Oliver-Vila I, Cabañas V, 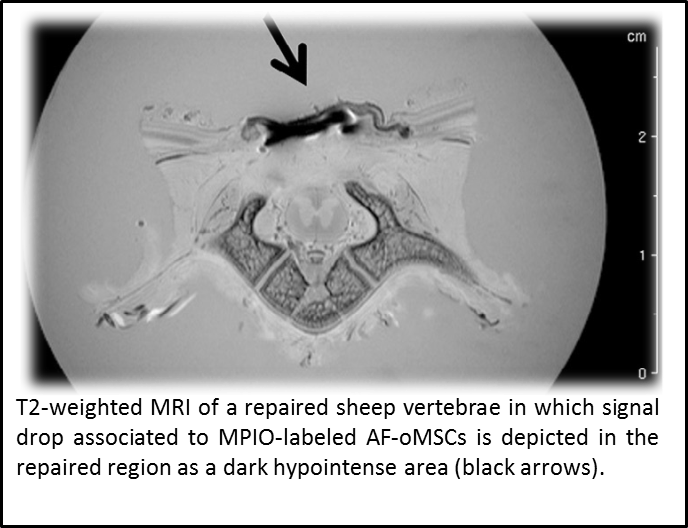
 “Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide” by by L. Ferrer-Font, N. Arias-Ramos,
“Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide” by by L. Ferrer-Font, N. Arias-Ramos, 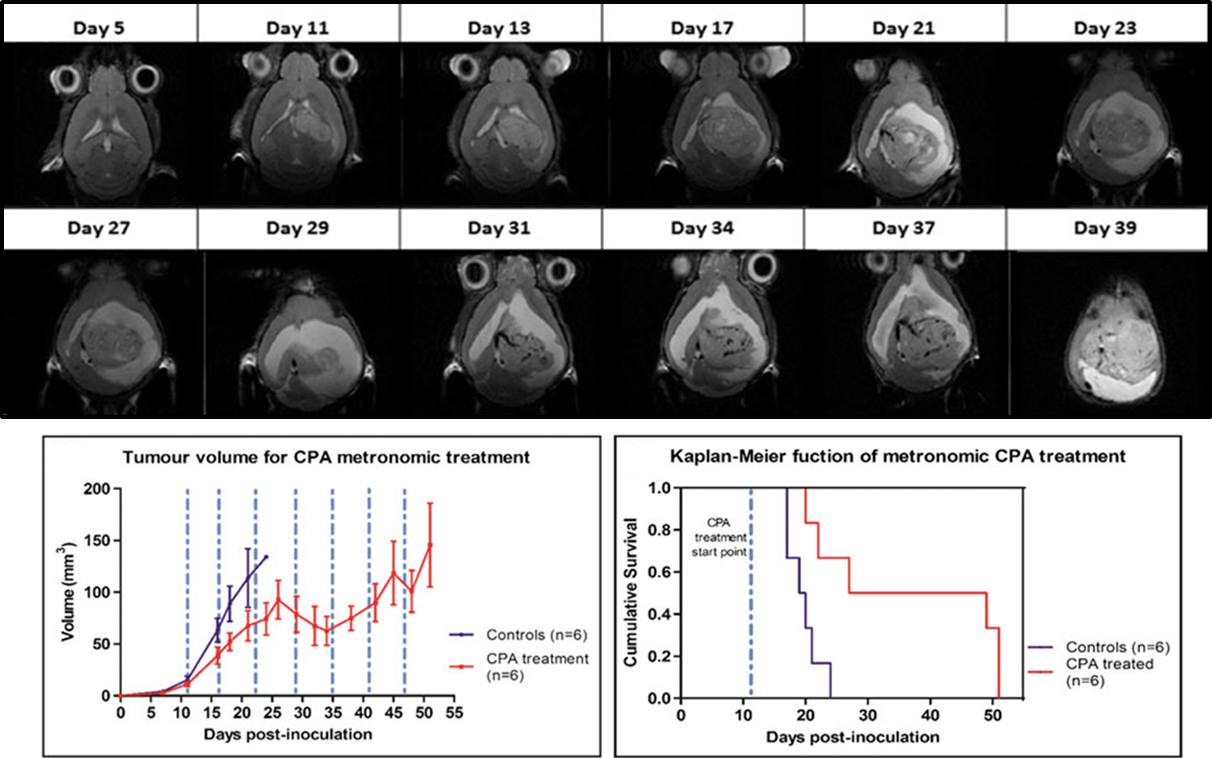

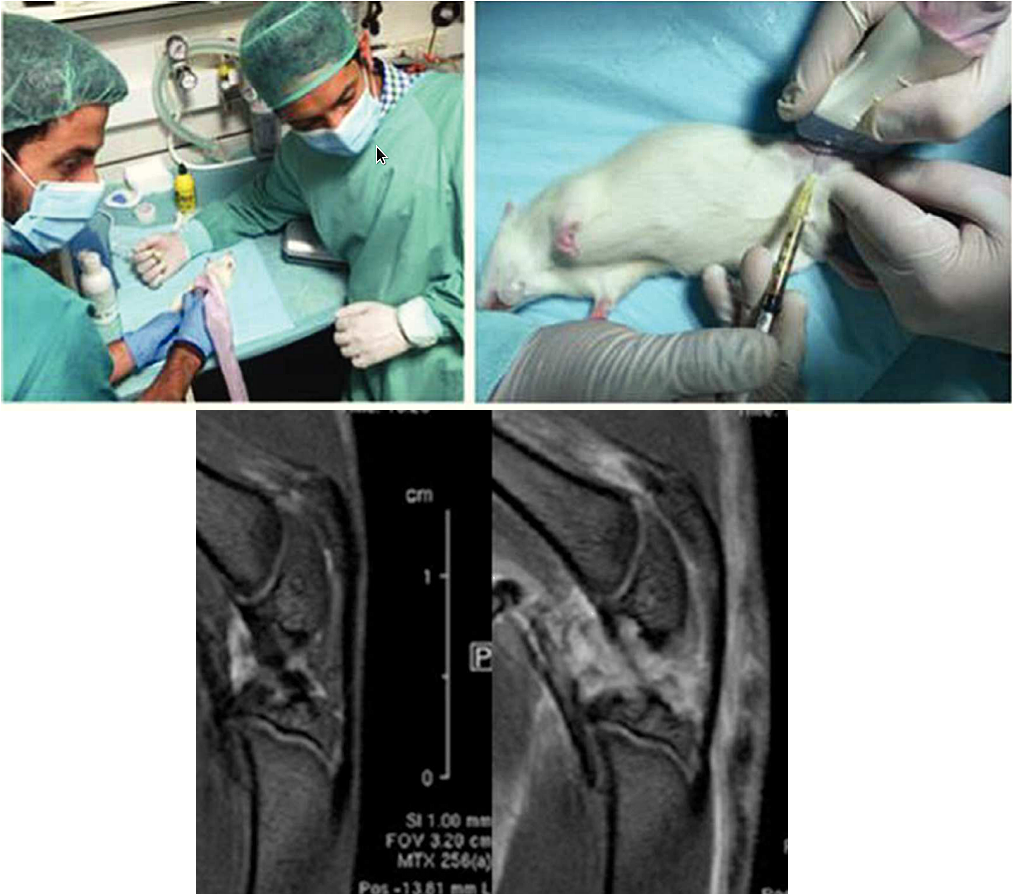
 “Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font,
“Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font, 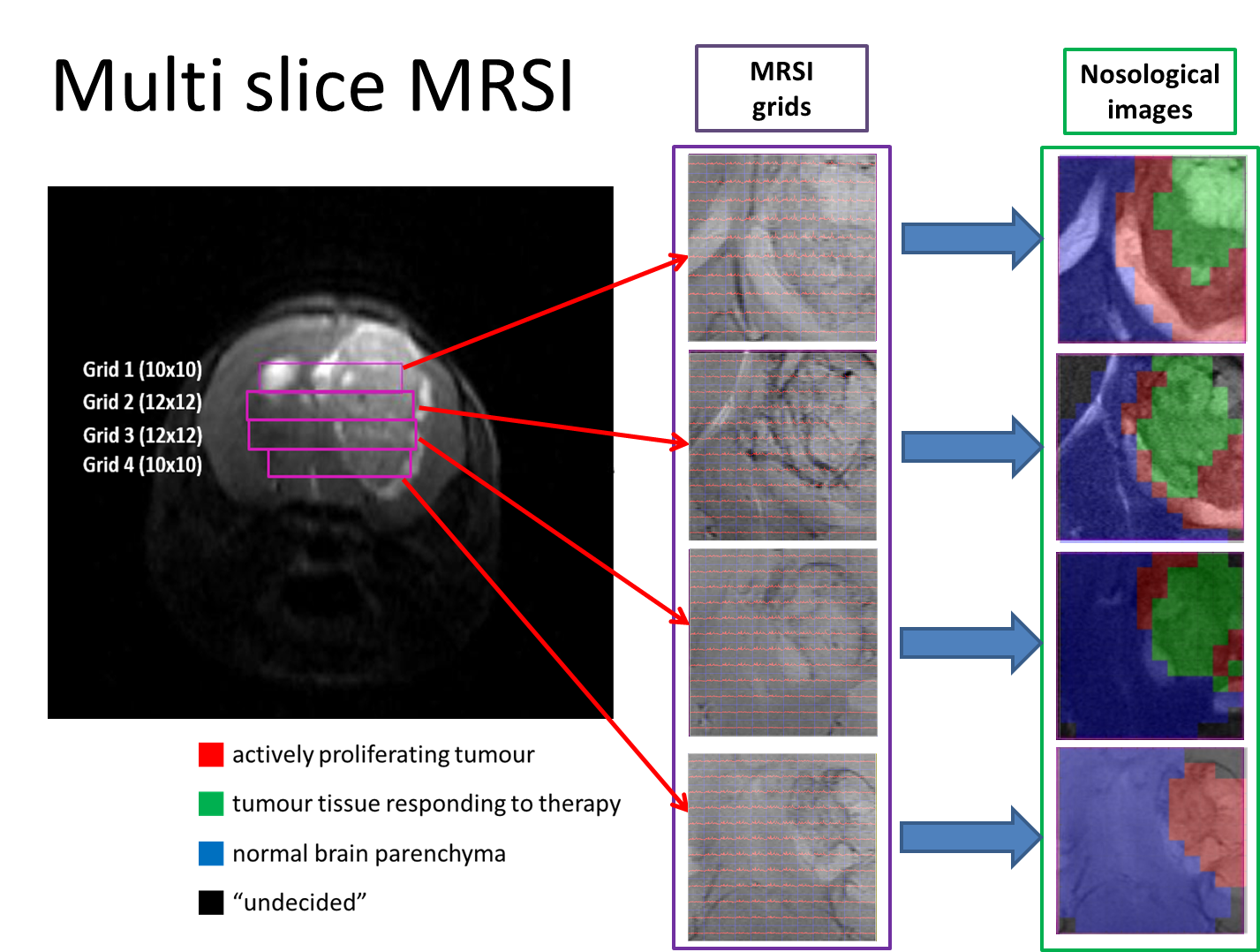 Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.
Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.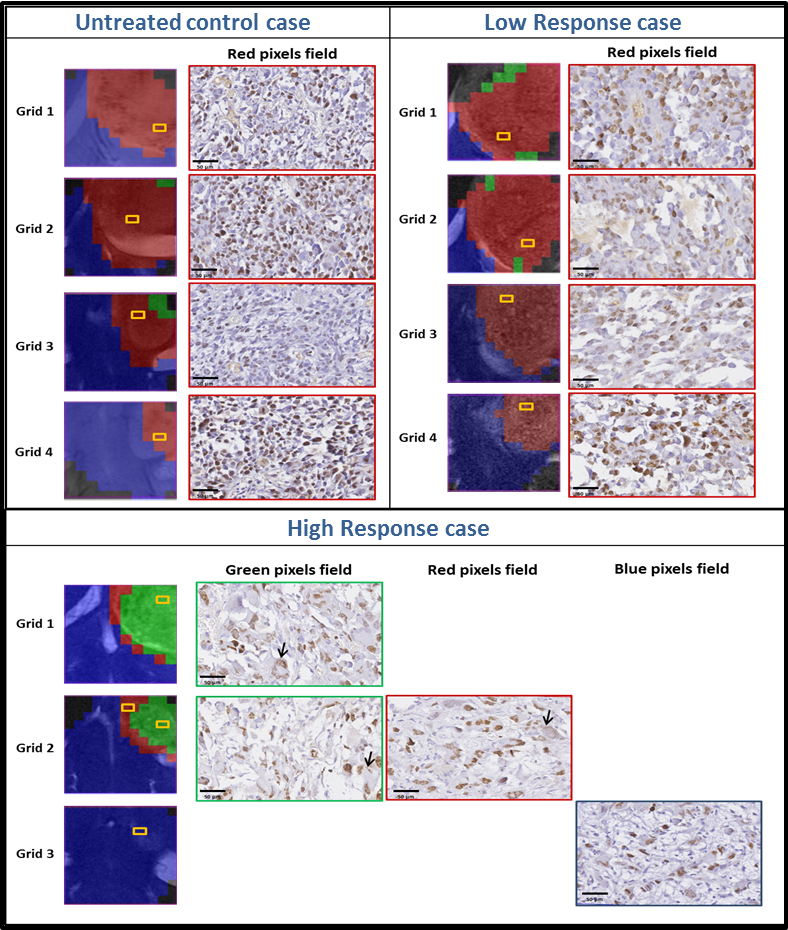
 “Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez,
“Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez, 
 “Mutation of the 3-Phosphoinositide-Dependent Protein Kinase 1
“Mutation of the 3-Phosphoinositide-Dependent Protein Kinase 1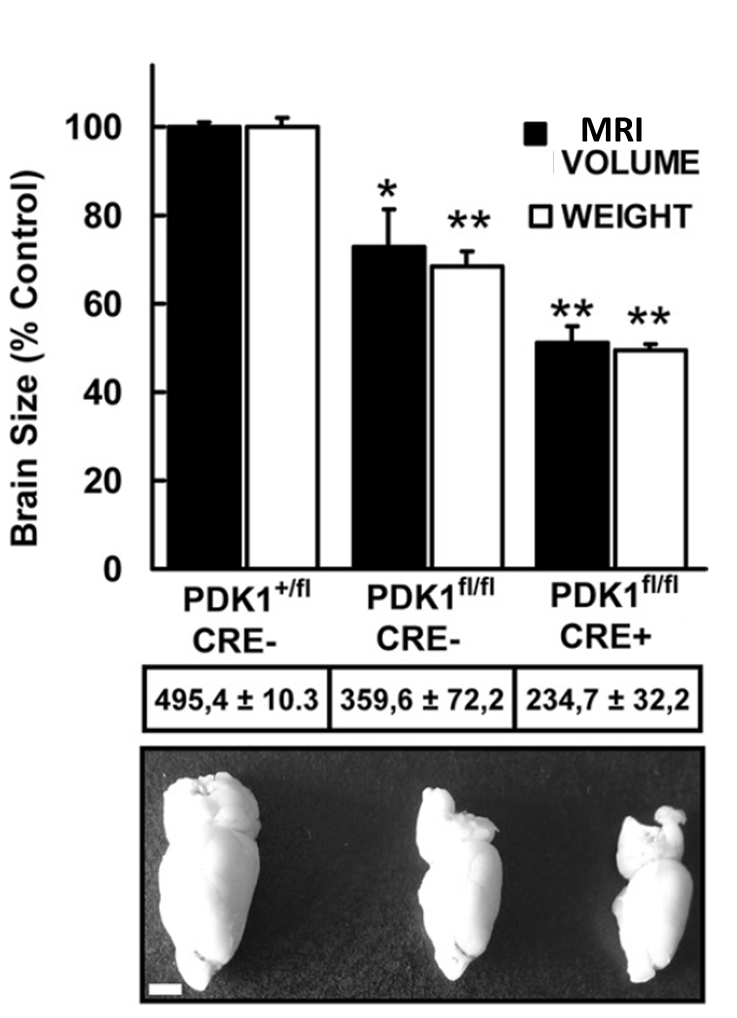 The phosphoinositide (PI) 3-kinase/Akt signaling pathway plays essential roles during neuronal development. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) coordinates the PI 3-kinase signals by activating 23 kinases of the AGC family, includingAkt. Phosphorylation of a conserved docking site in the substrate is a requisite for PDK1 to recognize, phosphorylate, and activate most of these kinases, with the exception of Akt. This differential mechanism of regulation it has been exploited by generating neuron-specific conditional knock-in mice expressing a mutant form of PDK1, L155E, in which the substrate-docking site binding motif, termed the PIF pocket, was disrupted. As a consequence, activation of all the PDK1 substrates tested except Akt was abolished. The mice exhibited microcephaly, altered cortical layering, and reduced circuitry, leading to cognitive deficits and exacerbated disruptive behavior combined with diminished motivation. The abnormal patterning of the adult brain arises from the reduced ability of the embryonic neurons to polarize and extend their axons, highlighting the essential roles that the PDK1 signaling beyond Akt plays in mediating the neuronal responses that regulate brain development.
The phosphoinositide (PI) 3-kinase/Akt signaling pathway plays essential roles during neuronal development. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) coordinates the PI 3-kinase signals by activating 23 kinases of the AGC family, includingAkt. Phosphorylation of a conserved docking site in the substrate is a requisite for PDK1 to recognize, phosphorylate, and activate most of these kinases, with the exception of Akt. This differential mechanism of regulation it has been exploited by generating neuron-specific conditional knock-in mice expressing a mutant form of PDK1, L155E, in which the substrate-docking site binding motif, termed the PIF pocket, was disrupted. As a consequence, activation of all the PDK1 substrates tested except Akt was abolished. The mice exhibited microcephaly, altered cortical layering, and reduced circuitry, leading to cognitive deficits and exacerbated disruptive behavior combined with diminished motivation. The abnormal patterning of the adult brain arises from the reduced ability of the embryonic neurons to polarize and extend their axons, highlighting the essential roles that the PDK1 signaling beyond Akt plays in mediating the neuronal responses that regulate brain development.