Progression of Alzheimer’s disease and effect of scFv-h3D6 immunotherapy in the 3xTg-AD mouse model: An in vivo longitudinal study using Magnetic Resonance Imaging and Spectroscopy by Güell-Bosch J, Lope-Piedrafita S, Esquerda-Canals G, Montoliu-Gaya L, and Villegas S. NMR in Biomedicine 33(5):e4263; DOI: 10.1002/nbm.4263.
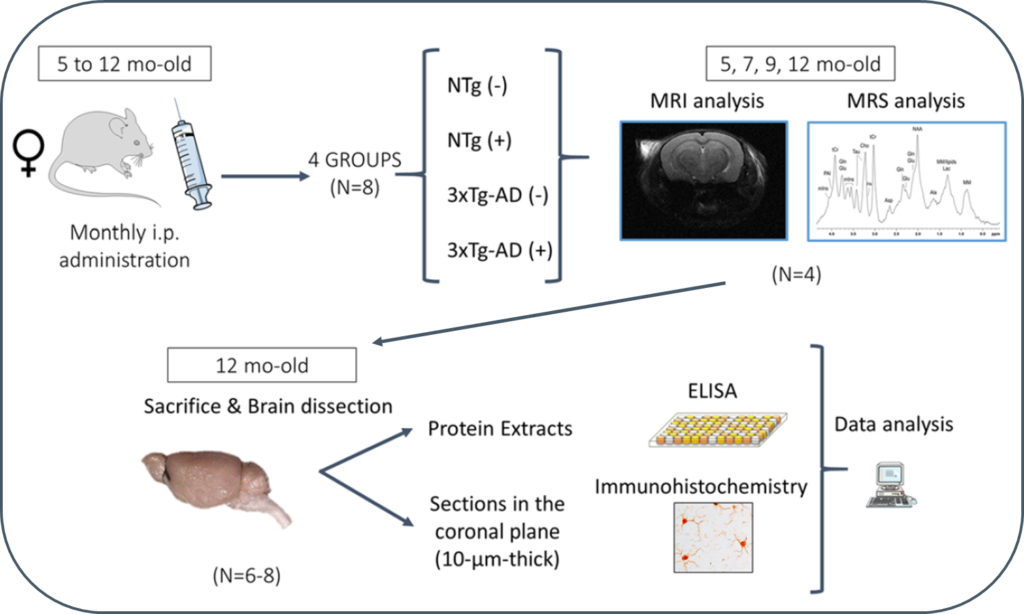
Alzheimer’s disease (AD) is an incurable disease that affects most of the 47 million people estimated as living with dementia worldwide. The main histopathological hallmarks of AD are extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein. In recent years, Aβ-immunotherapy has been revealed as a potential tool in AD treatment. One strategy consists of using single-chain variable fragments (scFvs), which avoids the fragment crystallizable (Fc) effects that are supposed to trigger a microglial response, leading to microhemorrhages and vasogenic edemas, as evidenced in clinical trials with bapineuzumab. The scFv-h3D6 generated by our research group derives from this monoclonal antibody, which targets the N-terminal of the Aβ peptide and recognizes monomers, oligomers and fibrils.
In this study, 3xTg-AD mice were intraperitoneally and monthly treated with 100 μg of scFv-h3D6 (a dose of ~3.3 mg/kg) or PBS, from 5 to 12 months of age (-mo), the age at which the mice were sacrificed and samples collected for histological and biochemical analyses. During treatments, four monitoring sessions using magnetic resonance imaging and spectroscopy (MRI/MRS) were performed at 5, 7, 9, and 12 months of age. MRI/MRS techniques allow, in a non-invasive manner, to draw an in vivo picture of concrete aspects of the pathology and to monitor its development across time. Compared with the genetic background, 3xTg-AD mice presented a smaller volume in almost all cerebral regions and ages examined, an increase in both the intra and extracellular Aβ1-42 at 12-mo, and an inflammation process at this age, in both the hippocampus (IL-6 and mIns) and cortex (IL-6). In addition, treatment with scFv-h3D6 partially recovered the values in brain volume, and Aβ, IL-6, and mIns concentrations, among others, encouraging further studies with this antibody fragment.
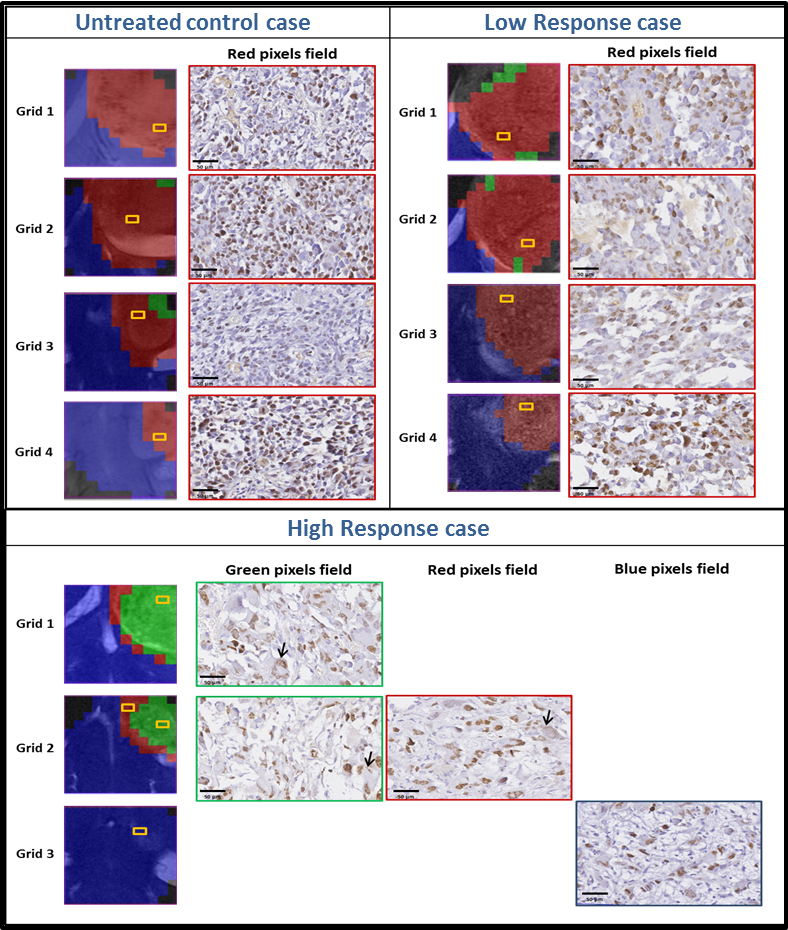
 “Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide” by by L. Ferrer-Font, N. Arias-Ramos,
“Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide” by by L. Ferrer-Font, N. Arias-Ramos, 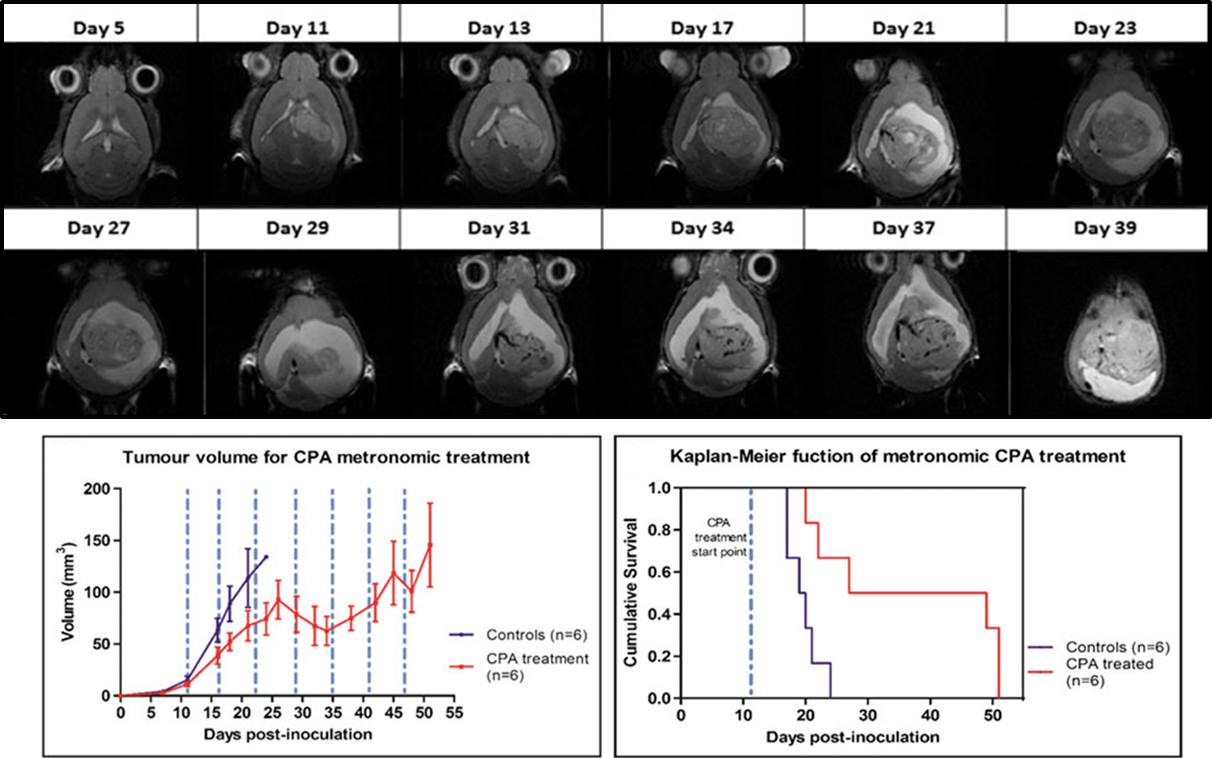
 “Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font,
“Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font, 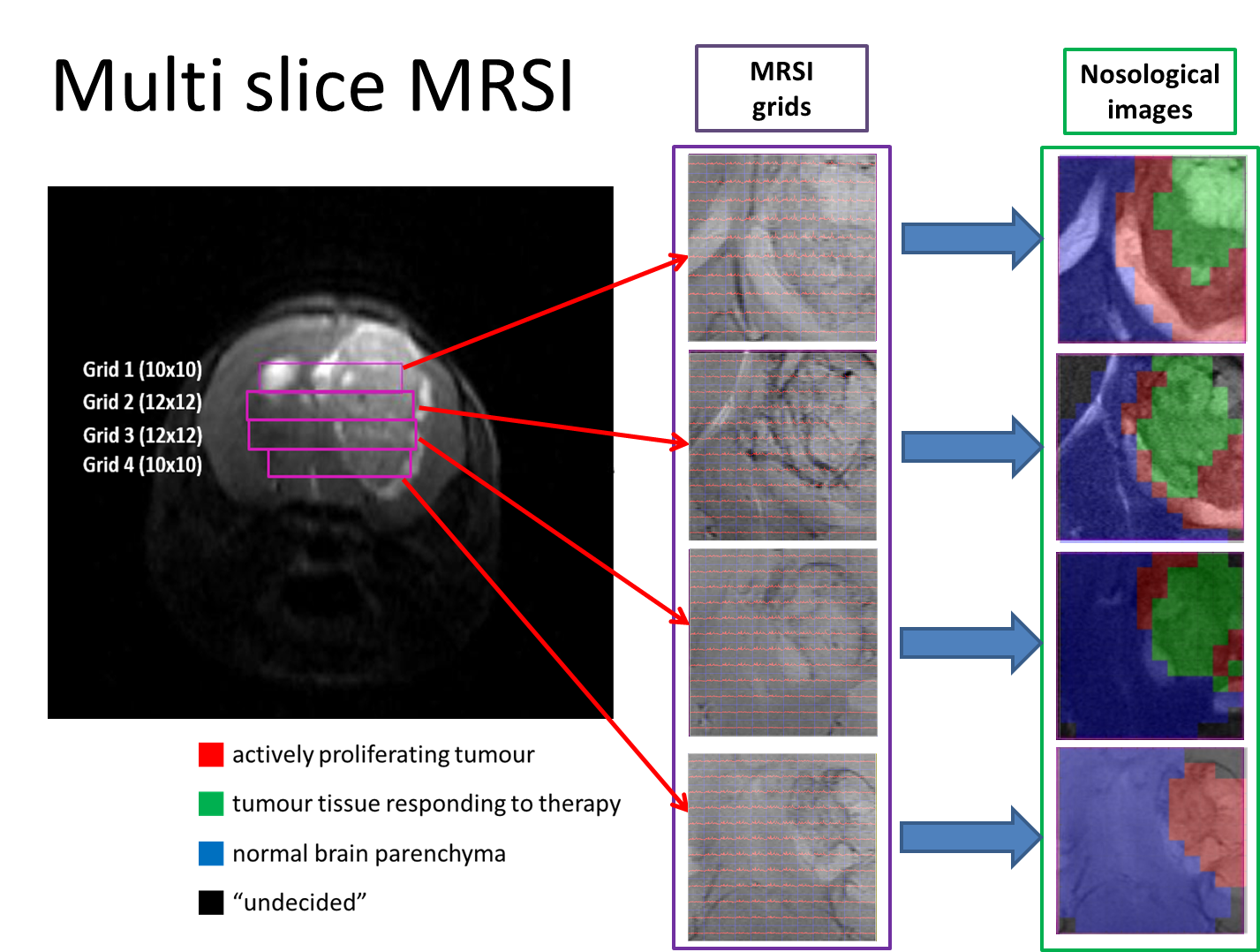 Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.
Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.