Stereoselectivity of Proline / Cyclobutane Amino Acid-Containing Peptide Organocatalysts for Asymmetric Aldol Additions: a Rationale
Stereoselectivity of Proline / Cyclobutane Amino Acid-Containing Peptide Organocatalysts for Asymmetric Aldol Additions: a Rationale
Tag Archives: peptides
Folding peptides studied by NMR
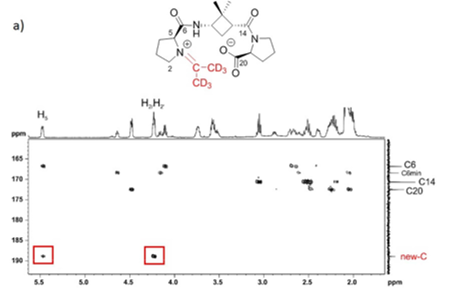
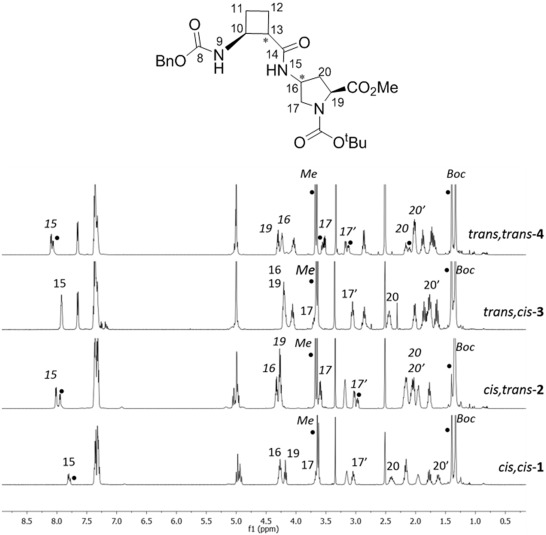
 The relevance of the relative configuration in the folding of hybrid peptides containing β-cyclobutane amino acids and γ-amino-L-proline residues
The relevance of the relative configuration in the folding of hybrid peptides containing β-cyclobutane amino acids and γ-amino-L-proline residues
O. Illa, J.A. Olivares, P. Nolis, R.M. Ortuño
DOI: 10.1016/j.tet.2017.09.011
Four new series of diastereomeric β,γ-di- and β,γ-tetrapeptides derived from conveniently protected (1R,2S)- and (1S,2S)-2-aminocyclobutane-1-carboxylic acid and cis- and trans-γ-amino-l-proline joined in alternation have been synthesized. High resolution NMR experiments show that peptides containing trans-cyclobutane amino acid residues adopt a more folded structure in solution than those containing a cis-cyclobutane residue, which adopt a strand-like structure. The cis/trans relative configuration of the cyclobutane residue is the origin of the folding pattern of each peptide due to either intra- or inter-residue hydrogen-bonded ring formation, whereas the cis/trans isomerism of the γ-amino-l-proline residue does not have a significantly relevant role on the folding ability of these peptides.
Gelation process followed by NMR
 Low-molecular-weight gelators consisting of hybrid cyclobutane-based peptides, by Sergi Celis, Pau Nolis, Ona Illa, Vicenç Branchadell, Rosa M. Ortuño, Organic & Biomolecular Chemistry 2013, 11, 2839 DOI: 10.1039/c3ob27347d
Low-molecular-weight gelators consisting of hybrid cyclobutane-based peptides, by Sergi Celis, Pau Nolis, Ona Illa, Vicenç Branchadell, Rosa M. Ortuño, Organic & Biomolecular Chemistry 2013, 11, 2839 DOI: 10.1039/c3ob27347dSome hybrid tetrapeptides consisting of (1R,2S)-2-aminocyclobutane-1-carboxylic acid and glycine, β-alanine, or γ-aminobutyric acid (GABA) joined in alternation, compounds 1–3, respectively, have been investigated to gain information on the non-covalent interactions responsible for their self-assembly to form ordered aggregates, as well as on parameters such as their morphology and size. All three peptides formed nice gels in many organic solvents and significant difference in their behaviour was not observed. Continue reading Gelation process followed by NMR
NMR confirms cysteine labelling with SFB in peptides
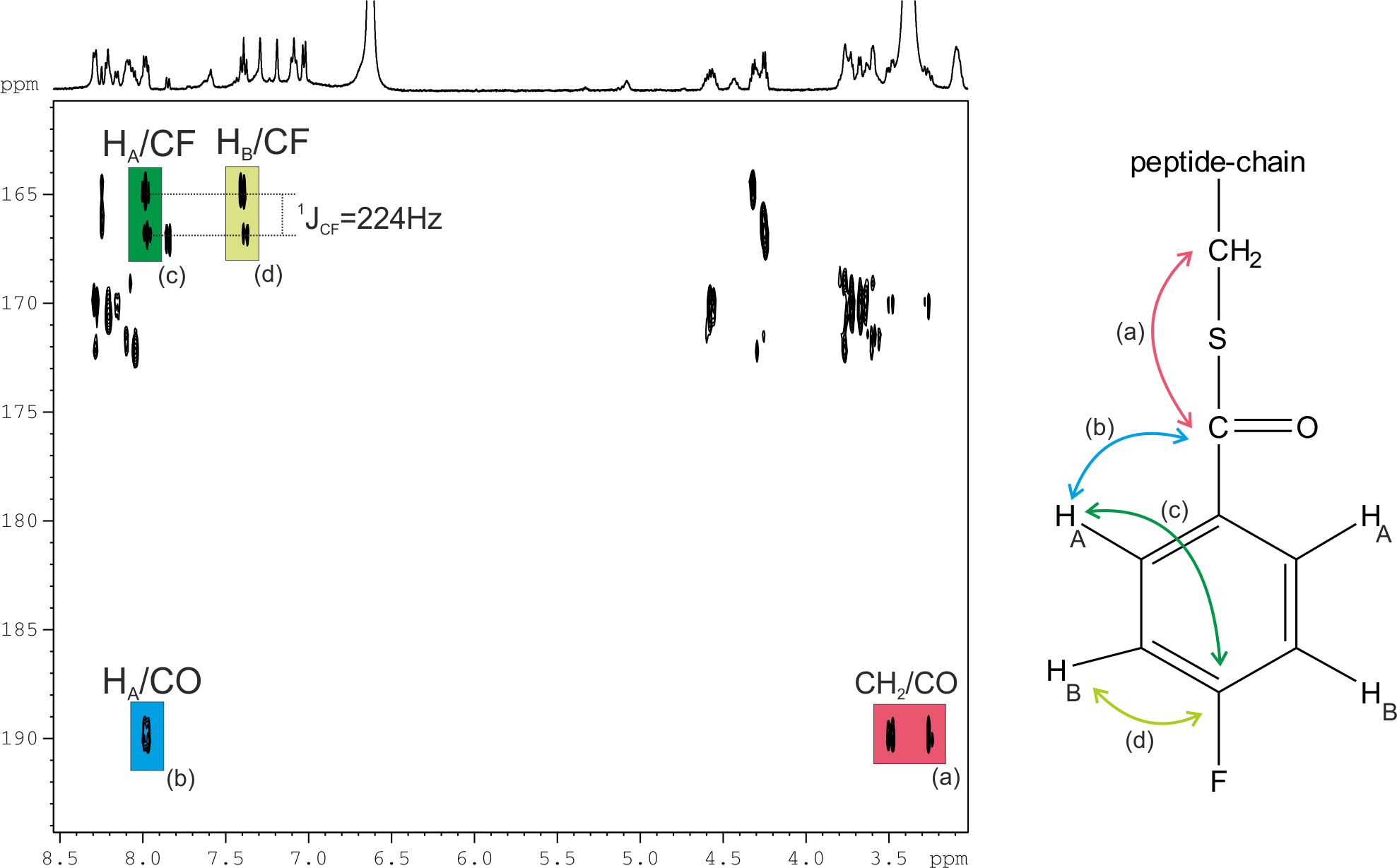
 “Efficient cysteine labelling of peptides with N-succinimidyl 4-[18F]fluorobenzoate: stability study and in vivo biodistribution in rats by positron emission tomography ( PET)” by Santiago Rojas, Pau Nolis, Juan D. Gispert, Jan Spengler, Fernando Albericio, José R. Herance, Sergio Abad. RSC Advances. 2013. DOI: 10.1039/C3RA40754C
“Efficient cysteine labelling of peptides with N-succinimidyl 4-[18F]fluorobenzoate: stability study and in vivo biodistribution in rats by positron emission tomography ( PET)” by Santiago Rojas, Pau Nolis, Juan D. Gispert, Jan Spengler, Fernando Albericio, José R. Herance, Sergio Abad. RSC Advances. 2013. DOI: 10.1039/C3RA40754C
A rapid and high-yielding cysteine labelling of peptides has been observed with the specific labelling agent for amines, N-succinimidyl 4-[18F]fluorobenzoate ([18F]SFB). Interestingly, NMR demonstrates that conjugation of the 4-fluorobenzoyl (FB) moiety is selectively achieved through a cysteine (Cys) thiol of the peptides with high yield (>80%) in short time (<5 min), while for a Cys amino acid derivative, a slow process has been observed. Continue reading NMR confirms cysteine labelling with SFB in peptides
Conformational Bias of Several Types of γ-Peptides
 “The Role of the Chiral cis-1,3-Disubstituted 2,2-Dimethylcyclobutane Motif in the Conformational Bias of Several Types of γ-Peptides” by Jordi Aguilera, Juan A. Cobos, Raquel Gutiérrez-Abad, Carles Acosta, Pau Nolis, Ona Illa, Rosa M. Ortuño. Eur. J. Org. Chem. 2013 (early view). DOI: 10.1002/ejoc.201300066
“The Role of the Chiral cis-1,3-Disubstituted 2,2-Dimethylcyclobutane Motif in the Conformational Bias of Several Types of γ-Peptides” by Jordi Aguilera, Juan A. Cobos, Raquel Gutiérrez-Abad, Carles Acosta, Pau Nolis, Ona Illa, Rosa M. Ortuño. Eur. J. Org. Chem. 2013 (early view). DOI: 10.1002/ejoc.201300066
Three series of new γ-peptides have been synthesized by starting from conveniently protected cis-3-amino-2,2-dimethylcyclobutane-1-carboxylic acid derivatives. The first series is constructed with only one enantiomer of this γ-amino acid, whereas in the second one both enantiomeric cyclobutane residues are joined in alternating fashion. Continue reading Conformational Bias of Several Types of γ-Peptides
Chiral secondary structure in β-peptides determined by NMR
 “Secondary Structure of Short β-Peptides as the Chiral Expression of Monomeric Building Units: a Rational and Predictive Model”Esther Gorrea, Gabor Pohl, Pau Nolis, Sergio Celis, Kepa K Burusco, Vicenç Branchadell, András Perczel, and Rosa M. Ortuño. Journal Of Organic Chemistry. ACCEPTED 2012 DOI: 10.1021/jo302034b
“Secondary Structure of Short β-Peptides as the Chiral Expression of Monomeric Building Units: a Rational and Predictive Model”Esther Gorrea, Gabor Pohl, Pau Nolis, Sergio Celis, Kepa K Burusco, Vicenç Branchadell, András Perczel, and Rosa M. Ortuño. Journal Of Organic Chemistry. ACCEPTED 2012 DOI: 10.1021/jo302034b
Chirality of the monomeric residues controls and determines the prevalent folding of small oligopeptides (from di- to tetramers) composed of the 2-aminocyclobutane-1-carboxylic acid (ACBA) derivatives with the same or different absolute and relative configuration. The cis-form of the monomeric ACBA gives rise to two conformers, namely Z6 and Z8, while the trans-form manifests uniquely as an H8 structure. Continue reading Chiral secondary structure in β-peptides determined by NMR
Novel MRI contrast agents: peptide-SPION conjugates
 “Efficient γ-amino-proline-derived cell penetrating peptide-superparamagnetic iron oxide nanoparticle conjugates via aniline-catalyzed oxime chemistry as bimodal imaging nanoagents”, by Cavalli S, Carbajo D, Acosta M, Lope-Piedrafita S, Candiota AP, Arús C, Royo M, Albericio F; Chem. Commun 48 (2012) p. 5322. DOI: 10.1039/C2CC17937G
“Efficient γ-amino-proline-derived cell penetrating peptide-superparamagnetic iron oxide nanoparticle conjugates via aniline-catalyzed oxime chemistry as bimodal imaging nanoagents”, by Cavalli S, Carbajo D, Acosta M, Lope-Piedrafita S, Candiota AP, Arús C, Royo M, Albericio F; Chem. Commun 48 (2012) p. 5322. DOI: 10.1039/C2CC17937G
Recent advances in nanotechnology have offered new opportunities for detection, prevention, and treatment of different diseases. In this respect engineered superparamagnetic iron oxide nanoparticles (SPIONs) represent an advanced tool in nanomedicine because they can be loaded in a multiple and orthogonal way with drugs and probes in order to simultaneously act as molecular imaging agents and drug carriers. Continue reading Novel MRI contrast agents: peptide-SPION conjugates

Cryoprobe: a key tool for the NMR characterization of small amount of peptides
 “Fast cysteine labelling of peptides promoted by an adjacent arginine has been observed with a standard labelling agent specific for amines, N-succinimidyl 4-[18F]fluorobenzoate” Sergio Abad, Pau Nolis, Juan D. Gispert, Jan Spengler, Fernando Albericio, Santiago Rojas and José R. Herance, ChemComm, Volume 48, pages 6118-6120, April 2012. DOI: 10.1039/C2CC32095A
“Fast cysteine labelling of peptides promoted by an adjacent arginine has been observed with a standard labelling agent specific for amines, N-succinimidyl 4-[18F]fluorobenzoate” Sergio Abad, Pau Nolis, Juan D. Gispert, Jan Spengler, Fernando Albericio, Santiago Rojas and José R. Herance, ChemComm, Volume 48, pages 6118-6120, April 2012. DOI: 10.1039/C2CC32095A
Fast cysteine labelling of peptides promoted by an adjacent arginine has been observed with a standard labelling agent specific for amines, N-succinimidyl 4-[18F]fluorobenzoate. HMBC experiment was the key experiment to corroborate this treat. The small amounts of some peptides herein studied required the sensitivty beneffits of a 500 MHz equipped with TCI cryprobe.
Designing hybrid foldamers
 “Designing hybrid foldamers: The effect on the peptide conformational bias of beta- versus alpha- and gamma-linear residues in alternation with (1R,2S)-2-aminocyclobutane-1-carboxylic acid” Sergi Celis, Esther Gorrea, Pau Nolis, Ona Illa, Rosa Maria Ortuño. Organic and Biomolecular Chemistry. Volume 10, Pages 861-868, October 2011 DOI: 10.1039/C1OB06575K
“Designing hybrid foldamers: The effect on the peptide conformational bias of beta- versus alpha- and gamma-linear residues in alternation with (1R,2S)-2-aminocyclobutane-1-carboxylic acid” Sergi Celis, Esther Gorrea, Pau Nolis, Ona Illa, Rosa Maria Ortuño. Organic and Biomolecular Chemistry. Volume 10, Pages 861-868, October 2011 DOI: 10.1039/C1OB06575K
Several oligomers constructed with (1R,2S)-2-aminocyclobutane-1-carboxylic acid and glycine, beta-alanine, and gamma-amino butyric acid (GABA), respectively, joined in alternation have been synthesized and studied by means of NMR and CD experiments as well as with computational calculations. Continue reading Designing hybrid foldamers
Structural study of γ,γ-peptides
 “Synthesis and structural study of highly constrained hybrid cyclobutane-proline γ,γ-peptides” by R. Gutiérrez-Abad, D. Carbajo, P. Nolis, C. Acosta-Silva, J. A. Cobos, O. Illa, M. Royo and R. Ortuño. Aminoacids, Volume 41, pages 673-686, 2011. DOI: 10.1007/s00726-011-0912-4.
“Synthesis and structural study of highly constrained hybrid cyclobutane-proline γ,γ-peptides” by R. Gutiérrez-Abad, D. Carbajo, P. Nolis, C. Acosta-Silva, J. A. Cobos, O. Illa, M. Royo and R. Ortuño. Aminoacids, Volume 41, pages 673-686, 2011. DOI: 10.1007/s00726-011-0912-4.
Two diastereomeric series of hybrid γ,γ-peptides derived from conveniently protected derivatives of (1R,2S)- and (1S,2R)-3-amino-2,2-dimethylcyclobutane-1-carboxylic acid and cis-4-amino-l-proline joined in alternation have efficiently been prepared through convergent synthesis. Continue reading Structural study of γ,γ-peptides