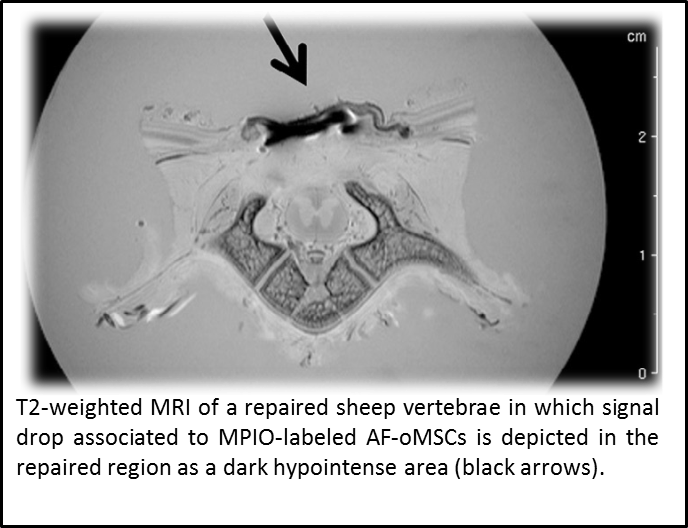
 “Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies” by Reyes B, Coca MI, Codinach M, López-Lucas MD, Del Mazo-Barbara A, Caminal M, Oliver-Vila I, Cabañas V, S. Lope-Piedrafita, García-López J, Moraleda JM, Fontecha CG, Vives J. Cytotherapy. 2017 :1060-1069. doi: 10.1016/j.jcyt.2017.06.004.
“Assessment of biodistribution using mesenchymal stromal cells: Algorithm for study design and challenges in detection methodologies” by Reyes B, Coca MI, Codinach M, López-Lucas MD, Del Mazo-Barbara A, Caminal M, Oliver-Vila I, Cabañas V, S. Lope-Piedrafita, García-López J, Moraleda JM, Fontecha CG, Vives J. Cytotherapy. 2017 :1060-1069. doi: 10.1016/j.jcyt.2017.06.004.
Biodistribution of candidate cell-based therapeutics is a critical safety concern that must be addressed in the preclinical development program. We aimed to design a decision tree based on a series of studies included in actual dossiers approved by competent regulatory authorities, noting that the design, execution and interpretation of pharmacokinetics studies using this type of therapy is not straightforward and presents a challenge for both developers and regulators. This work contributes to the standardization in the design of biodistribution studies by improving methods for accurate assessment of safety.
Eight studies were evaluated for the definition of a decision tree, in which mesenchymal stromal cells (MSCs) were administered to mouse, rat and sheep models using diverse routes (local or systemic), cell labeling (chemical or genetic) and detection methodologies (polymerase chain reaction (PCR), immunohistochemistry (IHC), fluorescence bioimaging, and magnetic resonance imaging (MRI). Moreover, labeling and detection methodologies were compared in terms of cost, throughput, speed, sensitivity and specificity.
 “Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide” by by L. Ferrer-Font, N. Arias-Ramos,
“Metronomic treatment in immunocompetent preclinical GL261 glioblastoma: effects of cyclophosphamide and temozolomide” by by L. Ferrer-Font, N. Arias-Ramos, 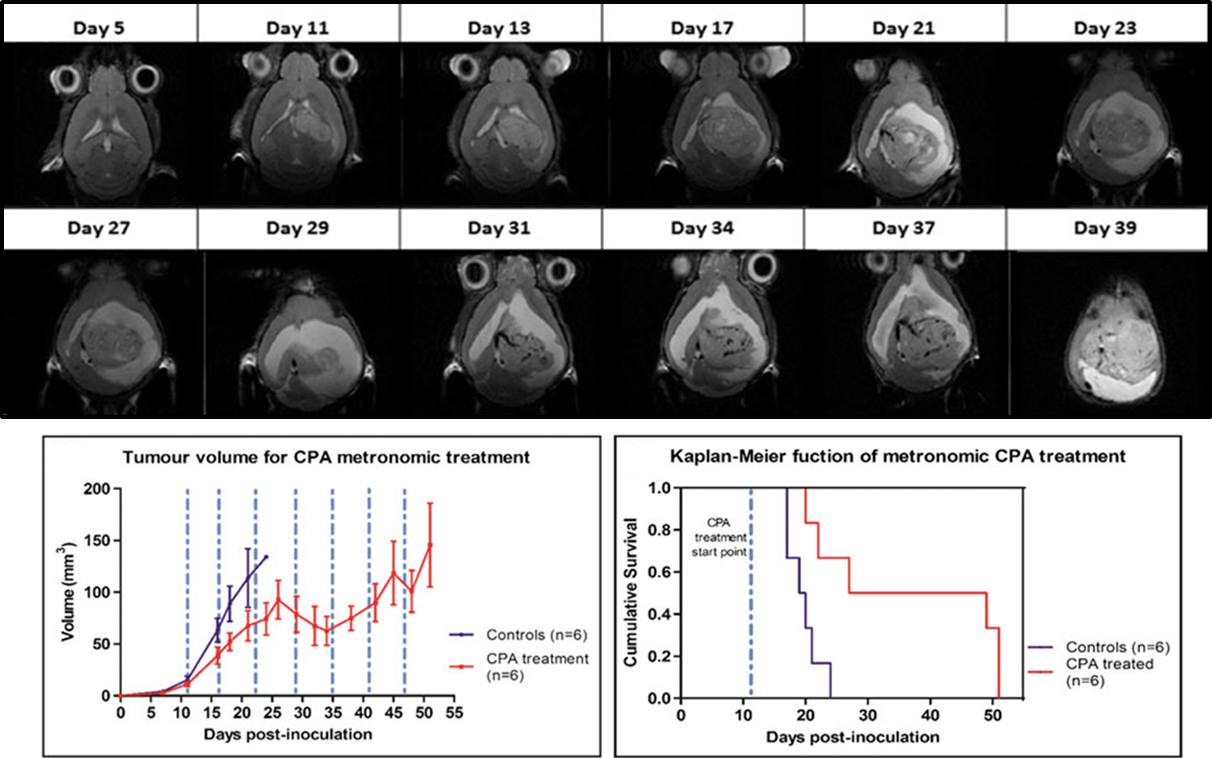

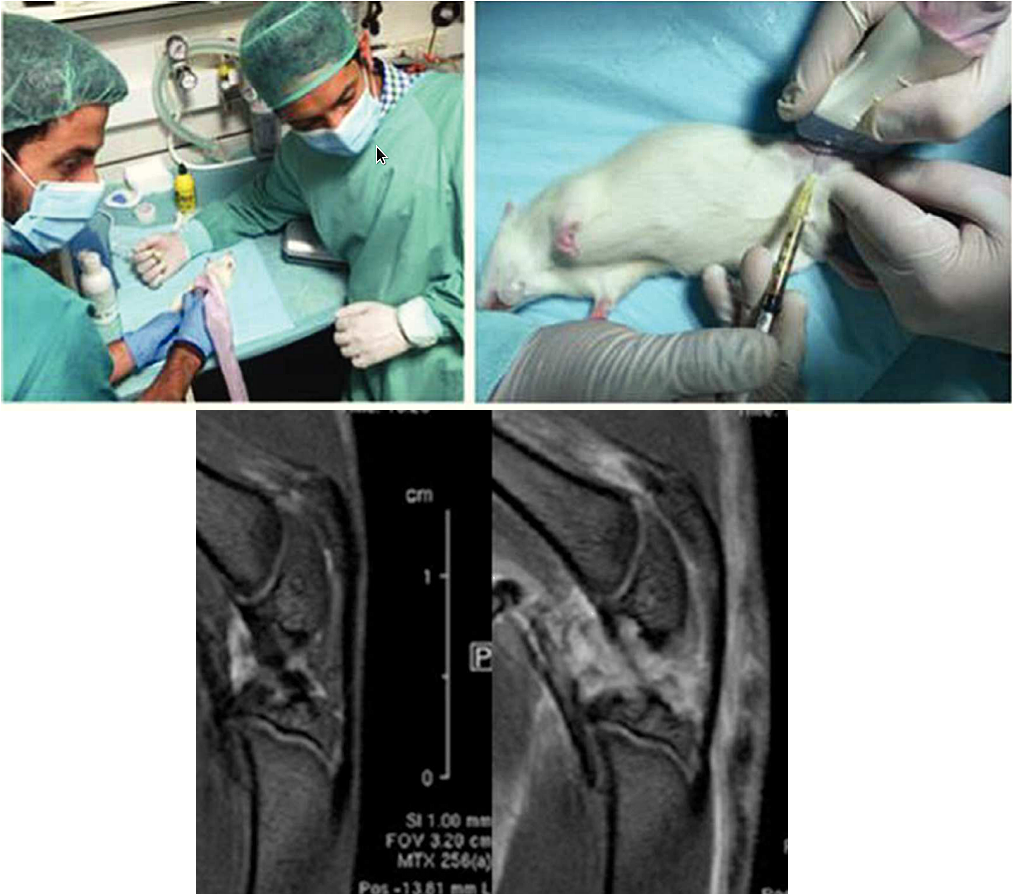
 “Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font,
“Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font, 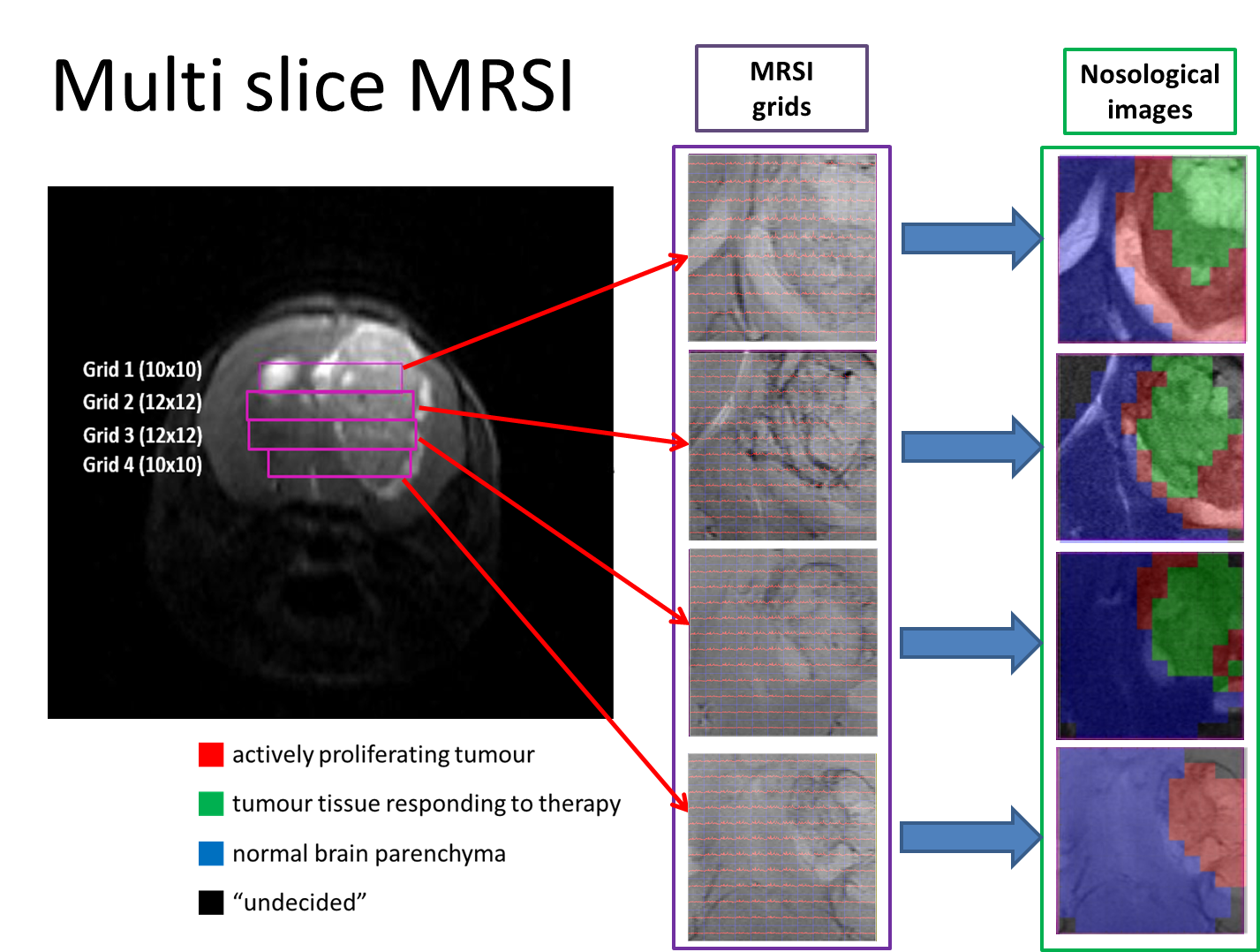 Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.
Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.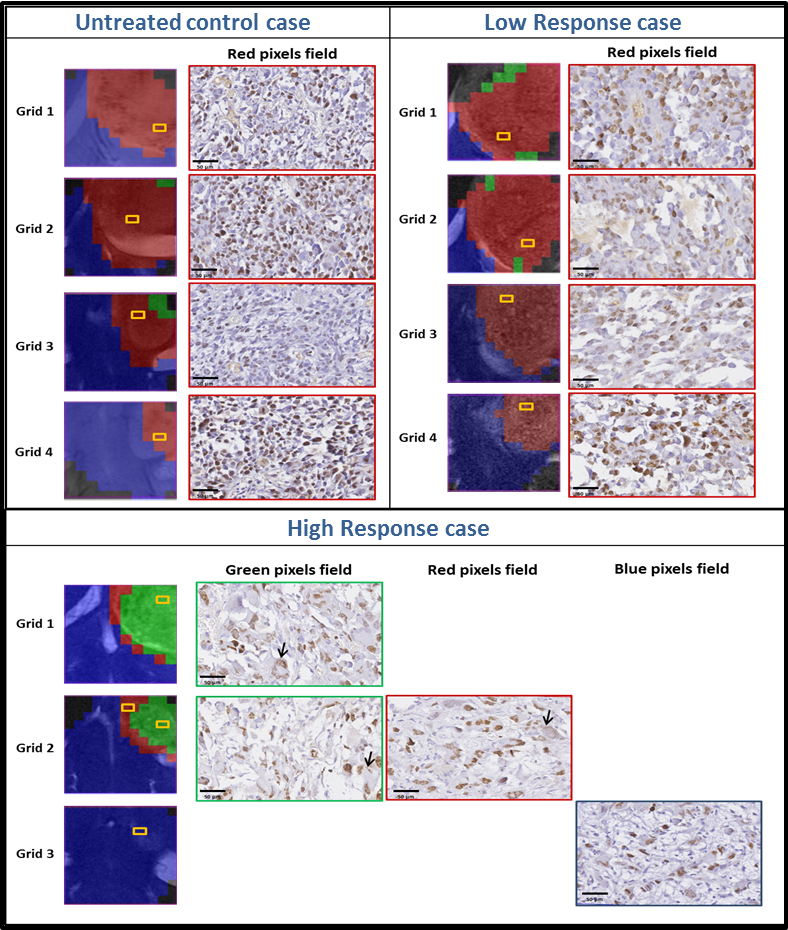
 “Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez,
“Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez, 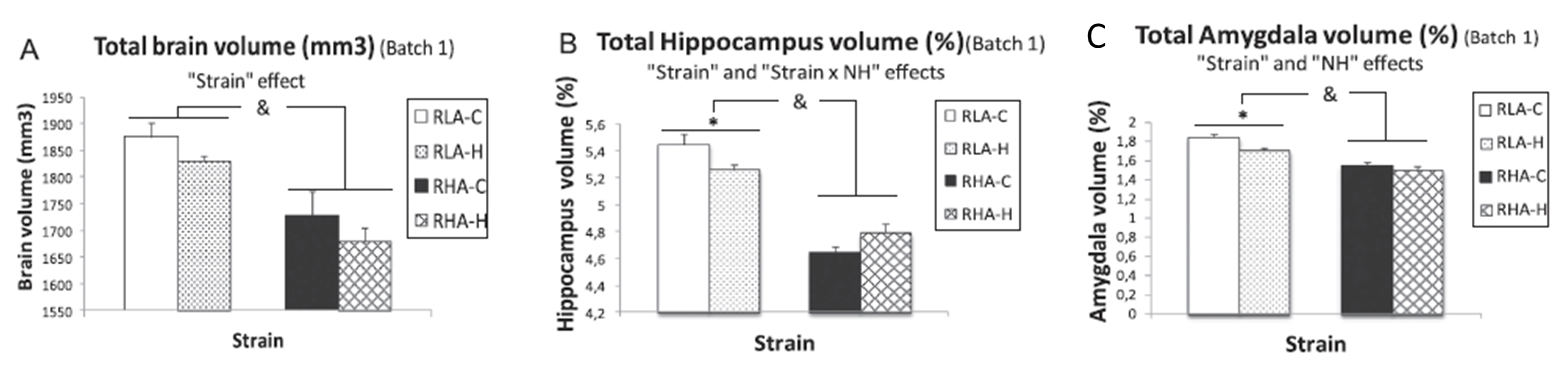
 “Mutation of the 3-Phosphoinositide-Dependent Protein Kinase 1
“Mutation of the 3-Phosphoinositide-Dependent Protein Kinase 1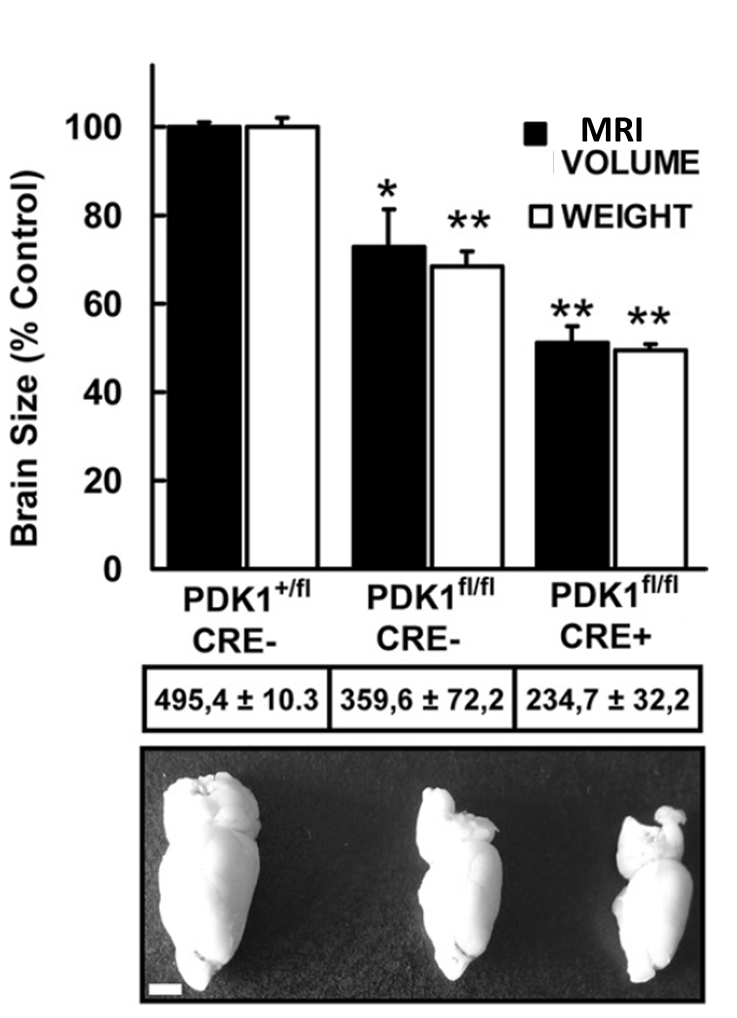 The phosphoinositide (PI) 3-kinase/Akt signaling pathway plays essential roles during neuronal development. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) coordinates the PI 3-kinase signals by activating 23 kinases of the AGC family, includingAkt. Phosphorylation of a conserved docking site in the substrate is a requisite for PDK1 to recognize, phosphorylate, and activate most of these kinases, with the exception of Akt. This differential mechanism of regulation it has been exploited by generating neuron-specific conditional knock-in mice expressing a mutant form of PDK1, L155E, in which the substrate-docking site binding motif, termed the PIF pocket, was disrupted. As a consequence, activation of all the PDK1 substrates tested except Akt was abolished. The mice exhibited microcephaly, altered cortical layering, and reduced circuitry, leading to cognitive deficits and exacerbated disruptive behavior combined with diminished motivation. The abnormal patterning of the adult brain arises from the reduced ability of the embryonic neurons to polarize and extend their axons, highlighting the essential roles that the PDK1 signaling beyond Akt plays in mediating the neuronal responses that regulate brain development.
The phosphoinositide (PI) 3-kinase/Akt signaling pathway plays essential roles during neuronal development. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) coordinates the PI 3-kinase signals by activating 23 kinases of the AGC family, includingAkt. Phosphorylation of a conserved docking site in the substrate is a requisite for PDK1 to recognize, phosphorylate, and activate most of these kinases, with the exception of Akt. This differential mechanism of regulation it has been exploited by generating neuron-specific conditional knock-in mice expressing a mutant form of PDK1, L155E, in which the substrate-docking site binding motif, termed the PIF pocket, was disrupted. As a consequence, activation of all the PDK1 substrates tested except Akt was abolished. The mice exhibited microcephaly, altered cortical layering, and reduced circuitry, leading to cognitive deficits and exacerbated disruptive behavior combined with diminished motivation. The abnormal patterning of the adult brain arises from the reduced ability of the embryonic neurons to polarize and extend their axons, highlighting the essential roles that the PDK1 signaling beyond Akt plays in mediating the neuronal responses that regulate brain development.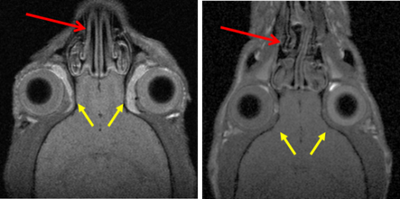

 ombines a comprehensive series of lectures on the technology of Magnetic resonance spectroscopy and imaging (MRS/MRI) with hands-on laboratory sessions to provide practical demonstrations of key concepts and procedures for preclinical studies.
ombines a comprehensive series of lectures on the technology of Magnetic resonance spectroscopy and imaging (MRS/MRI) with hands-on laboratory sessions to provide practical demonstrations of key concepts and procedures for preclinical studies.