“Generation of a new model of patellar tendinopathy in rats which mimics the human sports pathology: A pilot study” by David Domínguez, Paola Contreras-Muñoz, Silvia Lope, Gil Rodas, G. and Mario Marotta. Apunts. Medicina de l’Esport, 2017, 52:194, 53-59. DOI: 10.1016/j.apunts.2017.01.002
“Generation of a new model of patellar tendinopathy in rats which mimics the human sports pathology: A pilot study” by David Domínguez, Paola Contreras-Muñoz, Silvia Lope, Gil Rodas, G. and Mario Marotta. Apunts. Medicina de l’Esport, 2017, 52:194, 53-59. DOI: 10.1016/j.apunts.2017.01.002
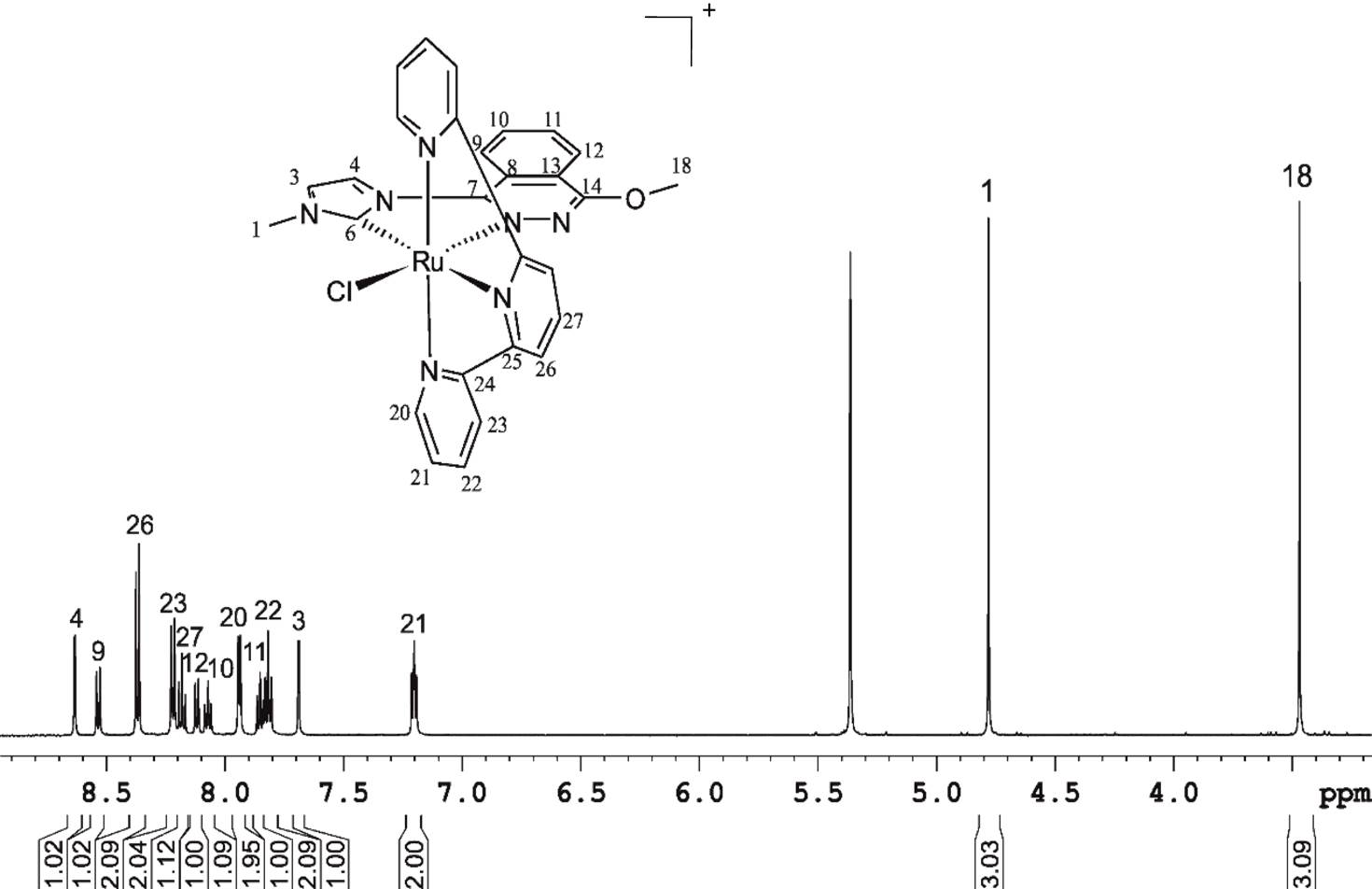
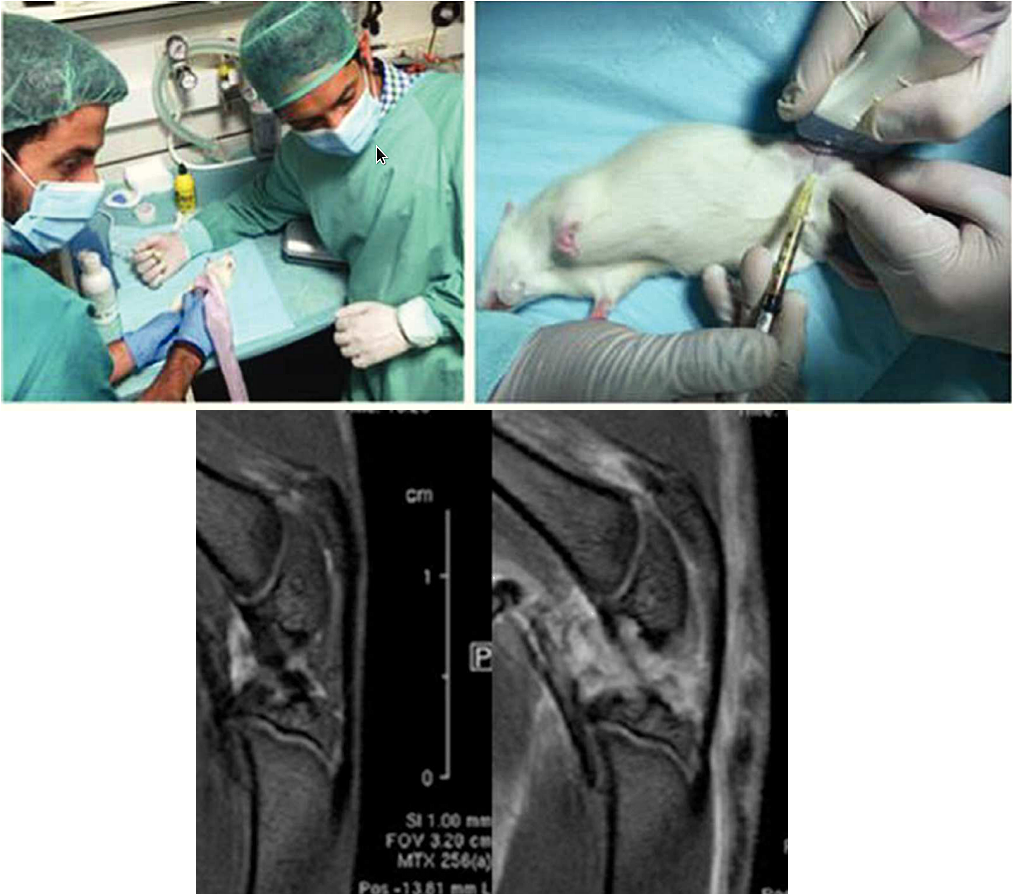
Introduction: Patellar tendon pathophysiology is not still fully understood. The collection of clinical samples from athletes that could permit the analysis of the tendinopathy progression, especially in the early stages, is difficult. For that reason, the purpose of this study is to develop a new experimental animal model of patellar tendinopathy in rats which mimics the human tendinopathy by in vivo intratendinous collagenase injection in the proximal portion of the patellar tendon. Material and methods: The experimental model used was 8-week-old male Wistar rats (N = 4). The administration of collagenase was performed by ultrasound-guided puncture at the level of the proximal and deep portion of the patellar tendon in anesthetized animals. The tendon lesion was evaluated 48 h after injury by magnetic resonance and then, the animals were euthanized and the patellar tendons were collected for histological evaluation. Results: The collagenase-induced lesion model demonstrated important similarities with the human patellar tendinopathy in the region of the proximal insertion. Conclusions: The experimental model of patellar tendinopathy in rat model induces a degeneration and distortion of the patellar tendon architecture in its proximal portion, which closely mimics to that seen in human patellar tendinopathy, and could represent an excellent preclinical model for the study of new therapies focused on treatment of tendinopathy.
 “Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font,
“Metabolomics of Therapy Response in Preclinical Glioblastoma: A Multi-Slice MRSI-Based Volumetric Analysis for Noninvasive Assessment of Temozolomide Treatment” by N. Arias-Ramos, L. Ferrer-Font, 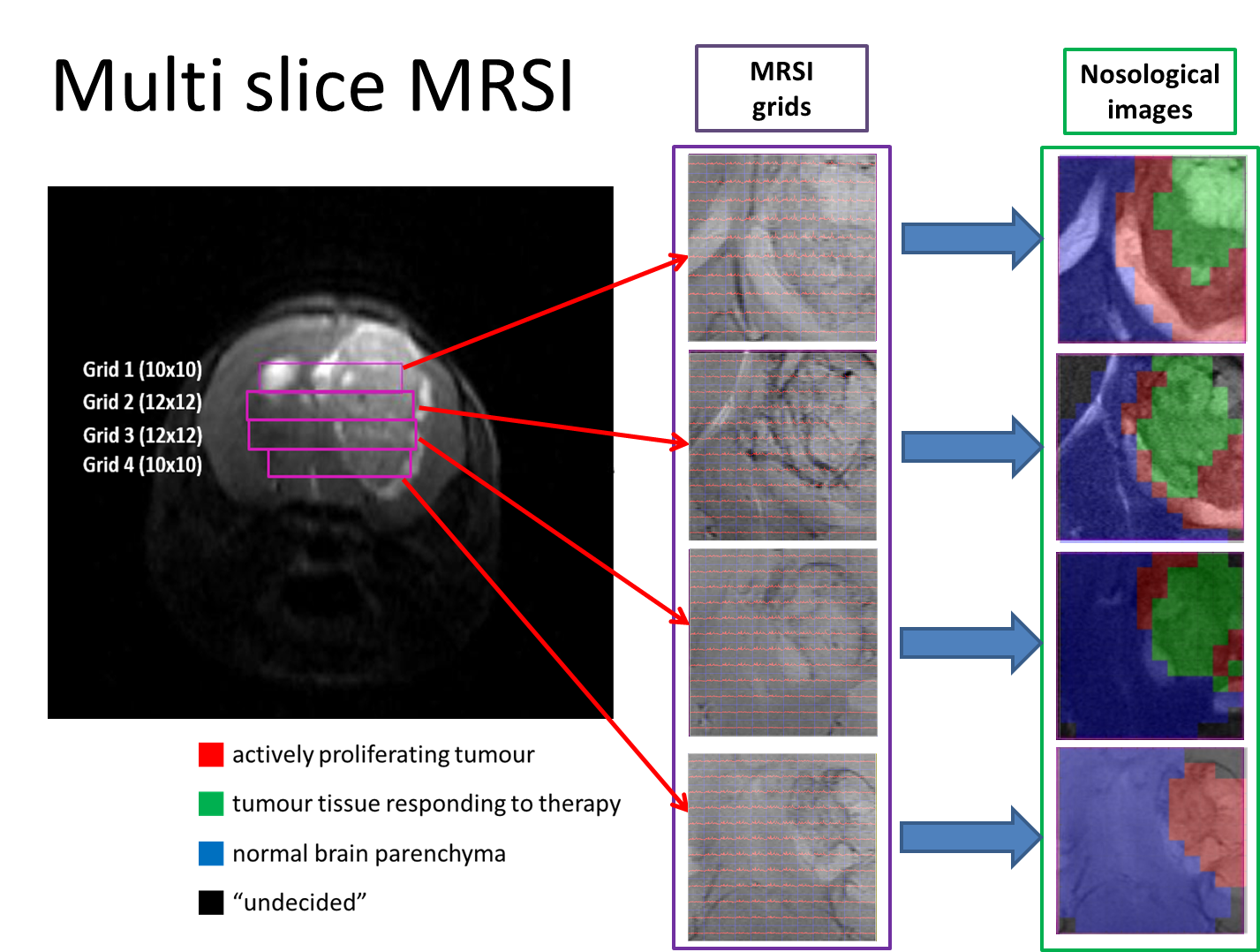 Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.
Therefore, the goal of this work was to acquire 3D-like information from preclinical GBM under a longitudinal treatment protocol, using a multi-slice MRSI approach.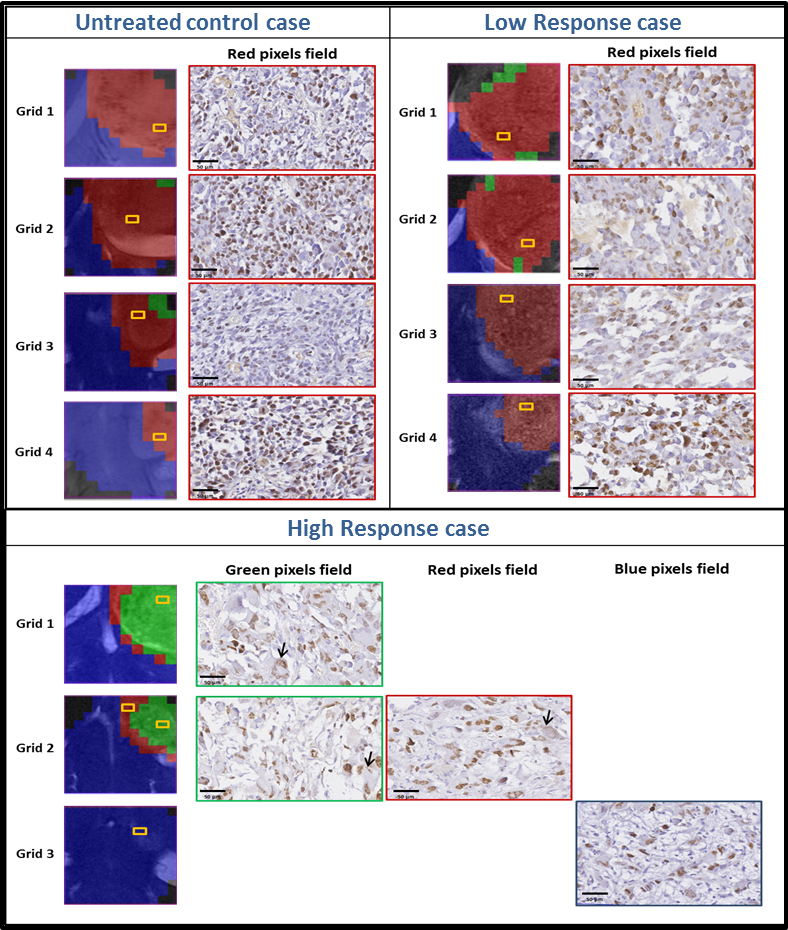




 “Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez,
“Neonatal handling enduringly decreases anxiety and stress responses and reduces hippocampus and amygdala volume in agenetic model of differential anxiety: Behavioral-volumetric associations in the Roman rats trains” by C. Río-Álamos, I. Oliveras, M. A. Piludu, C. Gerbolés, T. Cañete, G. Blázquez, 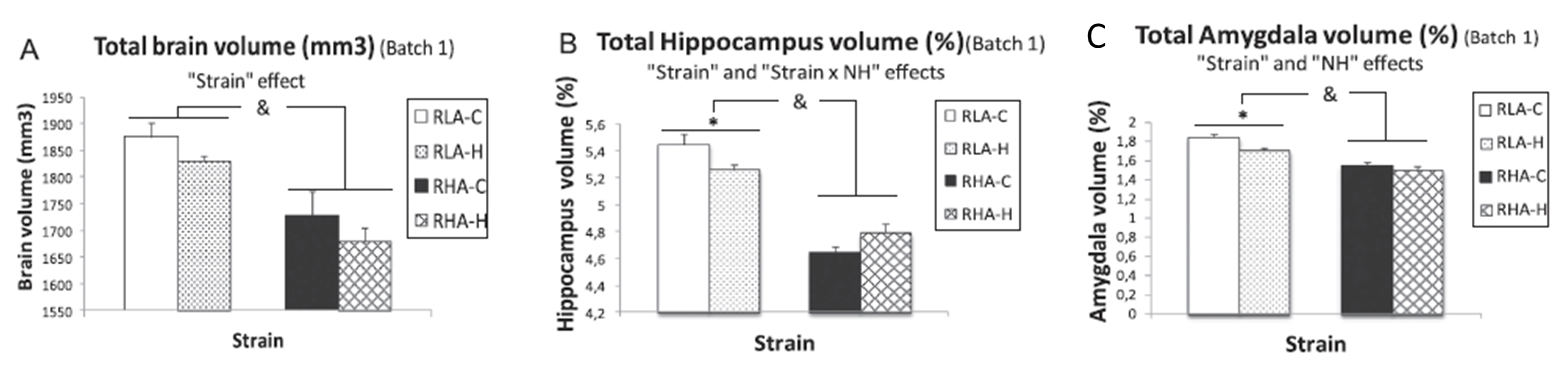
 “Mutation of the 3-Phosphoinositide-Dependent Protein Kinase 1
“Mutation of the 3-Phosphoinositide-Dependent Protein Kinase 1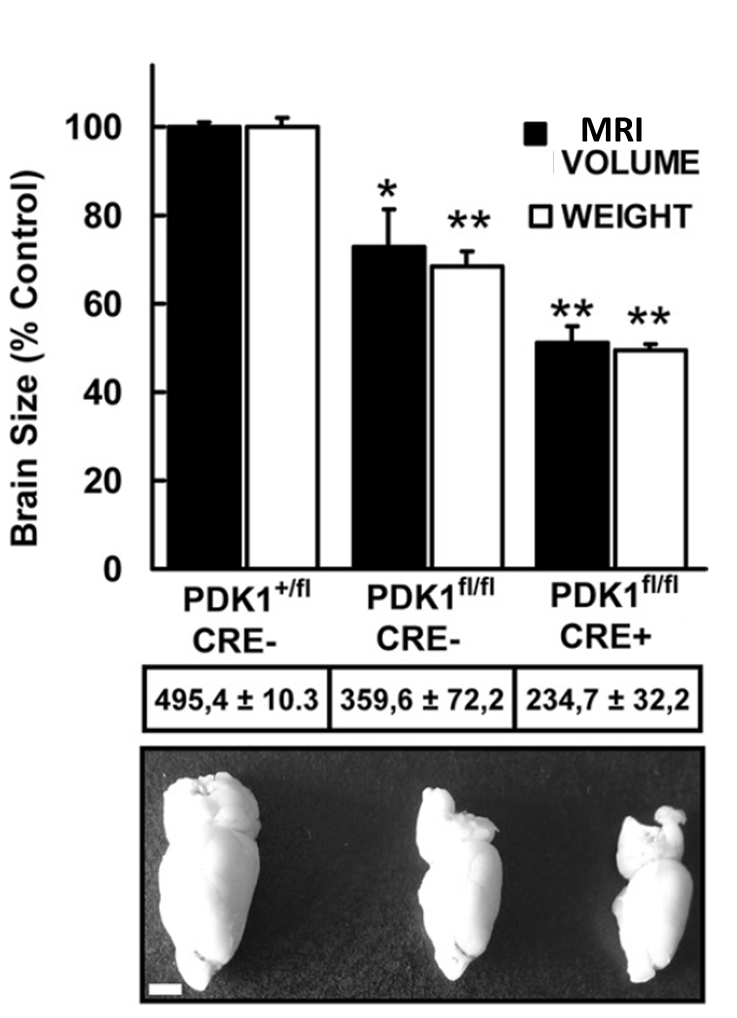 The phosphoinositide (PI) 3-kinase/Akt signaling pathway plays essential roles during neuronal development. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) coordinates the PI 3-kinase signals by activating 23 kinases of the AGC family, includingAkt. Phosphorylation of a conserved docking site in the substrate is a requisite for PDK1 to recognize, phosphorylate, and activate most of these kinases, with the exception of Akt. This differential mechanism of regulation it has been exploited by generating neuron-specific conditional knock-in mice expressing a mutant form of PDK1, L155E, in which the substrate-docking site binding motif, termed the PIF pocket, was disrupted. As a consequence, activation of all the PDK1 substrates tested except Akt was abolished. The mice exhibited microcephaly, altered cortical layering, and reduced circuitry, leading to cognitive deficits and exacerbated disruptive behavior combined with diminished motivation. The abnormal patterning of the adult brain arises from the reduced ability of the embryonic neurons to polarize and extend their axons, highlighting the essential roles that the PDK1 signaling beyond Akt plays in mediating the neuronal responses that regulate brain development.
The phosphoinositide (PI) 3-kinase/Akt signaling pathway plays essential roles during neuronal development. 3-Phosphoinositide-dependent protein kinase 1 (PDK1) coordinates the PI 3-kinase signals by activating 23 kinases of the AGC family, includingAkt. Phosphorylation of a conserved docking site in the substrate is a requisite for PDK1 to recognize, phosphorylate, and activate most of these kinases, with the exception of Akt. This differential mechanism of regulation it has been exploited by generating neuron-specific conditional knock-in mice expressing a mutant form of PDK1, L155E, in which the substrate-docking site binding motif, termed the PIF pocket, was disrupted. As a consequence, activation of all the PDK1 substrates tested except Akt was abolished. The mice exhibited microcephaly, altered cortical layering, and reduced circuitry, leading to cognitive deficits and exacerbated disruptive behavior combined with diminished motivation. The abnormal patterning of the adult brain arises from the reduced ability of the embryonic neurons to polarize and extend their axons, highlighting the essential roles that the PDK1 signaling beyond Akt plays in mediating the neuronal responses that regulate brain development.